Hypoxic-Ischemic Encephalopathy
Hypoxic ischemic encephalopathy (HIE) is one of the most serious birth complications affecting full term infants. HIE is a brain injury that prevents adequate blood flow to the infant’s brain occurring as a result of a hypoxic-ischemic event during the prenatal, intrapartum or postnatal period. By the age of 2 years, up to 60% of infants with HIE will die or have severe disabilities including mental retardation, epilepsy, and cerebral palsy (CP). The incidence of HIE has not declined even with advances in obstetric care (i.e. fetal monitoring) aimed at preventing the hypoxic-ischemic event.
While the exact cause of HIE is not always identified, antecedents include cord prolapse, uterine rupture, abruptio placenta, placenta previa, maternal hypotension, breech presentation, or shoulder dystocia. The manifestations of perinatal HIE in early postnatal life include abnormal fetal heart rate tracings, poor umbilical cord gases, low Apgar scores, presence of meconium stained fluid, or the need for respiratory support within the first several minutes of postnatal life. Health care providers also use the Sarnat staging criteria or an adapted version to describe the severity of encephalopathy within the first several postnatal days of life in conjunction with neuroimaging to assess the severity of the insult.
Epidemiology
Despite major advances in monitoring technology and knowledge of fetal and neonatal pathologies, HIE remains a serious condition that causes significant mortality and long-term morbidity.
United States data
In the United States and in most technologically advanced countries, the incidence of hypoxic-ischemic encephalopathy (HIE) is 1-4 cases per 1000 births.
International data
The incidence of HIE is reportedly high in countries with limited resources; however, precise figures are not available. Birth asphyxia is the cause of 23% of all neonatal deaths worldwide. It is one of the top 20 leading causes of burden of disease in all age groups (in terms of disability life adjusted years) by the World Health Organization and is the fifth largest cause of death of children younger than 5 years (8%). Although data are limited, birth asphyxia is estimated to account for 920,000 neonatal deaths every year and is associated with another 1.1 million intrapartum stillbirths. More than a million children who survive birth asphyxia develop problems such as cerebral palsy, mental retardation, learning difficulties, and other disabilities.
Race-, sex-, and age-related demographics
Most often, HIE is noted in infants who are term at birth. Preterm infants can also suffer from HIE, but the pathology and clinical manifestations are different. The symptoms of moderate-to-severe HIE are almost always manifested at birth or within a few hours after birth.
No race or sex predilection has been noted.
History
The guidelines from the American Academy of Pediatrics (AAP) and American College of Obstetrics and Gynecology (ACOG) for hypoxic-ischemic encephalopathy (HIE) indicate that all of the following must be present for the designation of perinatal asphyxia severe enough to result in acute neurologic injury:
- Profound metabolic or mixed acidemia (pH < 7) in an umbilical artery blood sample, if obtained
- Persistence of an Apgar score of 0-3 for longer than 5 minutes
- Neonatal neurologic sequelae (eg, seizures, coma, hypotonia)
- Multiple organ involvements (eg, kidney, lungs, liver, heart, intestines)
In rare instances, some babies will not fit the above criteria and the timing of the insult cannot be precisely known; however early magnetic resonance imaging of the brain can sometimes provide some insights.
Physical Examination – Signs and symptoms
CNS Manifestations
Clinical central nervous system (CNS) manifestations and course vary depending on hypoxic-ischemic encephalopathy (HIE) severity.
Mild hypoxic-ischemic encephalopathy
The infant seems hyperalert, muscle tone may be slightly decreased initially, and deep tendon reflexes may be brisk during the first few days.
Transient behavioral abnormalities, such as poor feeding, irritability, or excessive crying or sleepiness (typically in an alternating pattern), may be observed.
Typically resolves in less than 24 hours without any consequences.
Moderately severe hypoxic-ischemic encephalopathy
The infant is lethargic, with significant hypotonia and diminished deep tendon reflexes.
The grasping, Moro, and sucking reflexes may be sluggish or absent.
The infant may experience occasional periods of apnea.
Seizures typically occur early within the first 24 hours after birth.
Full recovery within 1-2 weeks is possible and is associated with a better long-term outcome.
An initial period of well-being or mild hypoxic-ischemic encephalopathy may be followed by sudden deterioration, suggesting ongoing brain cell dysfunction, injury, and death; during this period, seizure intensity might increase.
Severe hypoxic-ischemic encephalopathy
Stupor or coma is typical. The infant may not respond to any physical stimulus.
Breathing may be irregular, and the infant often requires ventilatory support.
Generalized hypotonia and depressed deep tendon reflexes are common.
Neonatal reflexes (eg, sucking, swallowing, grasping, Moro) are absent.
Disturbances of ocular motion, such as a skewed deviation of the eyes, nystagmus, bobbing, and loss of “doll’s eye” (ie, conjugate) movements may be revealed by cranial nerve examination.
Pupils may be dilated, fixed, or poorly reactive to light.
Seizures are delayed, can be severe and may be initially resistant to conventional treatments. The seizures are usually generalized, and their frequency may increase during the 24-48 hours after onset, correlating with the phase of reperfusion injury. As the injury progresses, seizures subside, and the EEG becomes isoelectric or shows a burst suppression pattern. At that time, wakefulness may deteriorate further, and the fontanelle may bulge, suggesting increasing cerebral edema.
Irregularities of heart rate and blood pressure (BP) are common during the period of reperfusion injury, as is death from cardiorespiratory failure.
Infants who survive severe hypoxic-ischemic encephalopathy
The level of alertness improves by days 4-5 of life.
Hypotonia and feeding difficulties persist, requiring tube feeding for weeks to months.
Neurologic Findings
Cranial nerves
Lack of reflex activity mediated by the cranial nerves can indicate brainstem dysfunction.
Full-term infants should blink and sustain eye closure in response to a sustained light stimulus. Repeated flashes of light should produce habituation (eg, attenuated blinking) after 3-4 stimuli. Virtually all full-term newborns can track a ball of red wool, and the movement of stripes of at least one eighth of an inch or bigger can elicit opticokinetic nystagmus. Objects and pictures with round contours and facial appearances also make good targets for tracking in the newborn. Tracking is possible in infants with complete destruction of the occipital cortex by virtue of a subcortical pulvinar-collicular system. Retinal hemorrhages are commonly observed in the neonate after vaginal delivery and can result in decreased pupil response. Destruction of the occipital cortex will also not affect pupillary response, because the responsible pathways leave the optic nerve and travel to the Edinger-Westphal nucleus, which sends back axons via the bilateral oculomotor nerves (consensual pupillary reflex).
Neurologic examination may be difficult in the small and frail premature infant, but weakness of the lower extremities sometimes reflects the neuropathologic substrate of periventricular leukomalacia. Over time, the patient with periventricular white-matter lesions develops spastic diplegia affecting the lower extremities more than the upper extremities.
Blinking to light starts at 26 weeks’ gestational age, sustained eye closure to light is seen around 32 weeks, and 90% of newborns track a ball of red wool by 34 weeks. Opticokinetic reflexes can be seen at 36 weeks. The pupil starts reacting to light around 30 weeks, but the light reflex is not consistently assessable until the gestational age of 32-35 weeks. Pupillary reflexes are reliably present at term. Extraocular movements can be elicited by performing the doll’s-eye maneuver at 25 weeks’ gestation and by performing caloric stimulation at 30 weeks’ gestation.
In infants aged 32-34 weeks’ gestation, suck and swallow are reasonably coordinated with breathing, but the actions are not perfected until after term.
Patients with mild HIE often have mydriasis. Progression of the disease may produce miosis (even in the dark) responsive to light, and in severe cases (stage 3 of Sarnat classification), the pupils are small or mid-positioned and poorly reactive to light, reflecting sympathetic or parasympathetic dysfunction.
The lack of pupillary, eye movement, corneal, gag, and cough reflexes may reflect damage to the brainstem, where the cranial-nerve nuclei are located. Decreased respiratory drive or apnea can be from lesions of the respiratory center, which overlap with vagal nuclei (ambiguous and solitaire) or medullary reticular formation. Ventilatory disturbances in HIE may manifest as periodic breathing apnea (similar to Cheyne-Stokes respiration) or just decreased respiratory drive.
Motor function
Begin the motor examination of an infant with suspected HIE by qualitatively and quantitatively observing his or her posture and spontaneous movements. Asymmetry in the amount of movement and posture is a subtle sign of hemiparesis, but it may be the only focal feature of the examination. Slight stimulation (eg, gently touching the patient) can increase motor activity in the term neonate and may be helpful in demonstrating asymmetrical hemiparesis.
Eliciting the Moro reflex may be an excessive stimulus and mask a subtle asymmetry in limb movement. Asymmetry in the Moro reflex is seen in peripheral lesions (eg, those due to brachial plexus injury).
Total absence or paucity of spontaneous movements, especially if associated with no reaction to painful stimuli and generalized hypotonia, indicates brainstem dysfunction or severe, diffuse, or multifocal cortical damage.
Specific patterns of motor weakness indicate cerebral injury patterns. Patients with borderzone parasagittal injury (ulegyria) tend to have proximal greater than distal weakness and upper extremity more than lower extremity weakness (man-in-the-barrel). A unilateral, focal infarct, especially one involving the middle cerebral artery, causes contralateral hemiparesis and focal seizures. Patients with selective neuronal necrosis may have severe hypotonia, stupor, and coma.
Motor examination of a newborn with large unilateral lesions may reveal mild hemiparesis and seizures in as many as 80%. The seizures are often partial (focal) and contralateral to the cortical lesion. Neonates with severe bilateral infarcts may have quadriparesis. Moro and tonic neck reflexes do not habituate, reflecting the lack of cortical modulation, which attenuates the response after repeated trials or sustained stimulus. Newborns with diencephalic lesions cannot regulate their temperature and have problems with sleep-wake cycles. The long-term sequelae of focal or multifocal cerebral necrosis include spastic hemiparesis and quadriparesis (eg, bilateral hemiparesis), cognitive deficits, and seizures.
Foot-ankle dorsiflexion or triple flexion (eg, foot-ankle dorsiflexion, knee and hip flexion) after plantar stimulation reflects only an intact spinal cord and sensory and motor nerves. Extensor movements (eg, arm elevation above the level of the shoulders) are more sophisticated motor actions than the dorsiflexion or triple flexion and require some cortical function.
A tonic neck reflex is performed by turning the patient’s head to one side. The patient demonstrates arm and leg extension on the side to which the head is turned and flexion on the opposite side (fencer’s posture). The tonic neck reflex posture should go away after several seconds, and its persistence is a sign of cortical dysfunction.
Spasticity is a velocity-dependent increase in tone that is generally most prominent with limb extension in muscle groups with antigravitational action (arm flexion, plantar extension). This sign can be seen over time in infants with corticospinal tract damage caused by a hypoxic-ischemic insult. In the neonatal period, spasticity is commonly noted first and is most prominent in the distal parts of the extremities. All fingers are flexed with the thumb under the second to fifth fingers, a pattern commonly referred to as cortical thumbs. Fewer than 5-10 beats of ankle clonus may be present in healthy neonates, but infants with damage to the corticospinal tract may have sustained ankle clonus. However, the initial motor manifestation will be flaccid hypotonia with spasticity later developing.
When assessing muscle tone, the state of arousal and prematurity must be considered. In the acute phase, tone is decreased in a generalized fashion affecting trunk and extremities. The flexor tone in the limbs is best assessed in term infants by showing a discrepancy in the scoring system between Dubowitz neurologic examination and morphologic examination. The infant looks like a “rag doll” when supported by a hand under the chest (vertical suspension). Head lag is demonstrated by traction of the hands in a supine position. The infant folds around the examiner’s hand when lifted prone with a hand supporting the chest (horizontal suspension).
Hip abduction may be seen with increased tone and even with decerebrate posturing (frog-leg posture). Another manifestation of CNS dysfunction in the neonatal period is increased axial extensor tone with arching of the back and neck extension or opisthotonus. Many infants simultaneously have decreased axial flexor tone (eg, major head lag on arm traction maneuver) and increased axial extensor tone. In many cases, limb and axial hypotonia are present for several months before increased axial extensor tone or limb spasticity can be detected. Increased active neck and trunk extensor tone are predictors of quadriparesis. Another sign of spasticity that can develop relatively early is scissoring, where the previously abducted legs extend, become rigid, and have extreme hip adduction such that they cross with stimulation or crying.
Seizures
HIE is often reported to be the most frequent cause of neonatal seizures. They usually occur 12-24 hours after birth and are difficult to control with anticonvulsants. Large, unilateral infarcts occur with neonatal seizures in as many as 80% of patients. Seizures are often partial (focal) and contralateral to the cortical lesion. About two thirds of newborns with cerebral venous infarcts have seizures. Those with multiple or diffuse lesions and cerebral venous infarcts often have multifocal or migratory seizures. Seizures are observed during physical examination and may confirm the diagnosis. Observation often reveals clonic rhythmic contractions. When holding the limb affected by clonic seizures, the examiner’s hand shakes or feels limb movement. Limb flexion or extension does not suppress the clonic activity, as it does in jitteriness and clonus. Newborn infants cannot have generalized seizures due to the immaturity of the neuronal pathways connecting the 2-halves of the brain.
Tonic, unilateral, or focal seizures consistently have an EEG signature. In the seizures, unilateral arm and leg posturing is often accompanied by ipsilateral trunk flexion. Generalized tonic posturing (eg, extension of the upper and lower extremities or extension of the legs and flexion of the arms) is related to an EEG seizure in 15% of affected neonates.
Tonic seizures can be seen in neonates with local anesthetic intoxication. Although generalized tonic posturing is infrequently associated with electrical seizures, it is not a benign sign. Of neonates with tonic posturing and an abnormal EEG background, 13% have normal development.
Mizrahi and Kellaway suggested the name brainstem release phenomena because tonic posturing and some subtle seizure-like motor automatisms are probably the result of primitive brainstem and spinal motor patterns liberated because the lack of inhibition from damaged forebrain structures. However, this tonic posturing is not a seizure and, thus, treatment with antiepileptics does not have benefit unless the infant is having other symptoms consistent with seizures.
Subtle seizures may be a part of the HIE picture. Subtle manifestations of neonatal seizures are confirmed on EEG and include apnea; tonic eye deviation; sustained eye opening; slow, rhythmic, tongue thrusting; and boxing, bicycling, and swimming movements. Most still accept that some subtle seizures may be correlated with EEG results. However, publications since the late 1980s have shown that seizures are not as frequent as previously thought and that they are unusual in patients close to term. Several other patterns of subtle neonatal seizures are described without EEG confirmation. The lack of an EEG signature does not exclude CNS pathology because neonates with HIE often have motor automatisms without EEG seizures. Management is controversial, but treatment is not usually beneficial unless more overt seizure activity is noted.
Seizures may be difficult to clinically diagnose in the premature neonate. Subtle seizures associated with ictal EEG changes are not rare in premature infants. The subtle patterns of neonatal seizures in the premature infant include sustained eye opening, oral-buccal-lingual movements (smacking, drooling, chewing), pedaling movements, grimacing, and autonomic manifestations.
Multiorgan Dysfunction
Multiorgan systems involvement is a hallmark of HIE. Organ systems involved in a hypoxic-ischemic event include the following:
Heart (43-78%)
May present as reduced myocardial contractility, severe hypotension, passive cardiac dilatation, and tricuspid regurgitation.
Lungs (71-86%)
Patients may have severe pulmonary hypertension requiring assisted ventilation.
Renal (46-72%)
Renal failure presents as oliguria and, during recovery, as high-output tubular failure, leading to significant water and electrolyte imbalances.
Liver (80-85%)
Elevated liver function test results, hyperammonemia, and coagulopathy can be seen. This may suggest possible GI dysfunction. Poor peristalsis and delayed gastric emptying are common; necrotizing enterocolitis is rare. Intestinal injuries may not be apparent in the first few days of life or until feeds are initiated.
Hematologic (32-54%)
Disturbances include increased nucleated RBCs, neutropenia or neutrophilia, thrombocytopenia, and coagulopathy. Severely depressed respiratory and cardiac functions and signs of brainstem compression suggest a life-threatening rupture of the vein of Galen (ie, great cerebral vein) with a hematoma in the posterior cranial fossa.
Sarnat Staging System
The staging system proposed by Sarnat and Sarnat in 1976 is often useful in classifying the degree of encephalopathy. Stages I, II, and III correlate with the descriptions of mild, moderate, and severe encephalopathy described above.
Table. Modified Sarnat Clinical Stages of Perinatal Hypoxic Ischemic Brain Injury
| MILD | MODERATE | SEVERE | |
| Level of Consciousness | Alternating (hyperalert, lethargic,irritable) | Lethargic or obtunded | Stuporous |
| Neuromuscular Control | |||
| Muscle tone | Normal | Hypotonia | Flaccid |
| Posture | Normal | Decorticate (arms flexed/legs extended) | Intermittent decerebration (arms and legs extended) |
| Stretch reflexes | Normal or hyperactive | Hyperactive or decreased | Absent |
| Segmental myoclonus | Present | Present | Absent |
| Complex Reflexes | |||
| Suck | Weak | Weak or absent | Absent |
| Moro | Strong; low threshold | Weak; incomplete; high threshold | Absent |
| Oculovestibular | Normal | Overactive | Weak or absent |
| Tonic neck | Slight | Strong | Absent |
| Autonomic Function | Generalized sympathetic | Generalized parasympathetic | Both systems depressed |
| Pupils | Mydriasis | Miosis | Variable; often unequal; poor light reflex |
| Heart Rate | Tachycardia | Bradycardia | Variable |
| Bronchial and Salivary Secretions | Sparse | Profuse | Variable |
| GI Motility | Normal or decreased | Increased; diarrhea | Variable |
| Seizures | None | Common; focal or multifocal | Delayed |
| EEG Findings | Normal (awake) | Early: low-voltage continuous delta and theta
Later: periodic pattern (awake) Seizures: focal 1-to 1-Hz spike-and-wave |
Early: periodic pattern with Isopotential phases
Later: totally isopotential |
| Duration | 1-3 days
Typically< 24h |
2-14 days | Hours to weeks |
Diagnostic Approach
Diagnostic investigations of infants with hypoxic-ischemic encephalopathy (HIE) are directed at establishing the severity and involvement of other organs, as well as to initiate an assessment of the prognosis. To that extent, initial laboratory testing should include determination of cardiac, renal and liver dysfunction.
In the era of therapeutic hypothermia, continuous video-electroencephalographic (EEG) monitoring is essential to assess encephalopathy severity and to monitor for seizures. Brain magnetic resonance imaging (MRI) is typically delayed until after rewarming is complete, although earlier MRI may be helpful in circumstances in which redirection of care is being considered.
Laboratory Studies
There are no specific tests to confirm or exclude a diagnosis of hypoxic-ischemic encephalopathy (HIE) because the diagnosis is made on the basis of the history, physical, neurologic examinations, and laboratory evidence. Many of these tests are performed to assess the likelihood of severe brain injury and to monitor the functional status of the organ systems. As always, the results of the tests should be interpreted in conjunction with the clinical history and the findings from the physical examination.
Laboratory studies should include the tests below.
Serum electrolyte levels
In severe cases, daily assessment of serum electrolytes is valuable until the infant’s status improves. Markedly low serum sodium, potassium, and chloride levels in the presence of reduced urine flow and excessive weight gain may indicate acute tubular damage or syndrome of inappropriate antidiuretic hormone (SIADH) secretion, particularly during the initial 2-3 days of life.
Similar changes may be seen during recovery; increased urine flow may indicate ongoing tubular damage and excessive sodium loss relative to water loss
Cardiac function studies
A cardiac enzymatic study gives an estimation of the extent of cardiac injury from asphyxia.
Renal function studies
Serum creatinine levels, creatinine clearance, and blood urea nitrogen (BUN) levels suffice in most cases.
Liver enzymes
These values are an adjunct to assess the degree of hypoxic-ischemic injury to these other organs. These findings may also provide some insight into injuries to other organs, such as the liver.
Coagulation system evaluation
This includes prothrombin time, partial thromboplastin time, fibrinogen levels, and serial platelet counts to assess the synthetic functions of the liver as well as assess for consumptive coagulopathy or bone marrow suppression.
Arterial blood gas (ABG)
Blood gas monitoring is used to assess acid-base status and to avoid hyperoxia and hypoxia as well as hypercapnia and hypocapnia. During the period of shock, capillary blood gases may not be reliable.
Imaging Studies
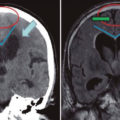
Brain magnetic resonance imaging (MRI)
MRI is the imaging modality of choice for the diagnosis and follow-up of infants with moderate-to-severe hypoxic-ischemic encephalopathy (HIE). Conventional MRI sequences (T1w and T2w) provide information on the status of myelination and preexisting developmental defects of the brain. When performed after the first day (and particularly after day 4), conventional images may accurately demonstrate the injury pattern as area of hyperintensity. Conventional images are most helpful at age 7-10 days, when the diffusion-weighted imaging (DWI) findings have pseudonormalized.
Following a severe asphyxial event, a central pattern of injury is seen with injury to (1) the deep gray matter (ie, putamina, ventrolateral thalamus, hippocampi, dorsal brainstem, or lateral geniculate nucleus) and (2) the perirolandic cortex. These areas contain the highest concentration of N-methyl-D-aspartate (NMDA) receptors and are actively myelinating.
Less severe or partial insult results in injury to the intervascular boundaries areas and is also called watershed injury. This type of lesions manifests in the infants as proximal extremity weakness or spasticity.
Decreased signal in the posterior limb of the internal capsule (PLIC) on T1w images may be noted. The absence of normal signal (high intensity on T1w images) in the PLIC of infants older than 38 weeks’ gestation is a strong predictor of abnormal motor outcomes in these infants.
DWI allows earlier identification of injury patterns in the first 24-48 hours. The MRI sequence identifies areas of edema and, hence, injured areas. DWI changes peak at 3-5 day and pseudonormalizes by the end of the first week. In neonates, DWI changes may underestimate the extent of injury, most likely because of the importance of apoptosis in the ultimate extent of neurologic injury.
MRI is also a useful tool in the determination of prognosis. Studies indicate that infants with predominant injuries to the basal ganglia or thalamus (BGT) have an unfavorable neurologic outcome when compared with infants with a white matter predominant pattern of injury. Abnormal signals in the PLIC have also been associated with poor neurologic outcome. In one study, severe BGT lesions on early MRI (performed at a median of 10 d; range, 2-42 d) were strongly associated with motor impairment at 2 years. In addition, abnormal PLIC signal was also highly correlated with inability to walk independently at 2 years, with a sensitivity of 0.92 and a specificity of 0.77.
In a study of MRIs at term-equivalent age from 3 cohorts of 325 very preterm infants, Kidokoro et al found 33% had some grade of brain injury (eg, periventricular leukomalacia, intraventricular/cerebellar hemorrhage) and 10% had severe brain injury. The investigators noted severe brain injury and impaired growth patterns were independently associated with perinatal risk factors and delayed cognitive development.
Both conventional images (T1- and T2-weighted) and diffusion techniques (DWI and ADC maps) have a good specificity (>90%) and positive predictive value (>85%) in predicting death or major disability at age 2 years. However, sensitivity and negative predictive values are low.
MRI is also useful for follow-up. In any newly diagnosed case of cerebral palsy, MRI should be considered because it may help in establishing the cause. Note that the interpretation of MRI in infants requires considerable expertise.
Magnetic resonance spectroscopy (MRS) allows for quantification of intracellular molecules. Proton MRS allows identification of cerebral lactate, which persist for weeks following a significant hypoxic-ischemic injury. Phosphorous MRS allows for real-time quantification of ATP, phosphorus creatinine, inorganic phosphorous, and intracellular pH levels.
Cranial ultrasonography
Although portable and convenient, cranial ultrasonography has a low sensitivity (50%) for the detection of anomalies associated with HIE. Findings include global increase in cerebral echogenicity and obliteration of cerebrospinal fluid (CSF) containing spaces suggestive of cerebral edema. Increase in the echogenicity of deep gray matter structures may also be identified, typically when ultrasonography is performed after 7 days of life. Finally, head ultrasonography has a very limited role to rule out intracerebral or intraventricular hemorrhages, and it is not very useful to learn the extent of brain injury.
In small studies, ultrasonography-based semi-quantitative markers such as the white matter/gray matter echogenicity ratio, as well as the resistive index, have emerged as potentially helpful tools to assess HIE severity and to assist in prognosis formulation early in the disease course. These studies indicate that in infants with HIE, white matter/gray matter echogenicity ratios are increased. Furthermore, neonates with a resistive index below 0.6 have an increased risk of neurodevelopmental impairment at age 20-32 months.
Head computed tomography (CT) scanning
Head CT scanning is a rapid mode of screening and is very effective in detecting hemorrhage with the added advantage of limited sedation need; however, evidence suggests that CT scan exposes children to potentially harmful radiation. Additionally, CT scanning is not a sensitive modality for evaluation of HIE because of the high-water content in the neonatal brain and the high protein content of the cerebrospinal fluid, which result in poor parenchymal contrast resolution. Because of these concerns and owing to the superiority of MRI in evaluating brain structures, head CT scanning is not recommended in the evaluation of neonates with HIE.
Echocardiography
Obtain an echocardiogram to evaluate the cardiac contractility and ejection fraction. Note that neonates with HIE receiving therapeutic hypothermia may experience a reduction in cardiac output and descending aorta blood flow. Systemic organ perfusion and cerebral metabolism may be affected by preferential cerebral distribution of cardiac output in conjunction with an increase in systemic peripheral vascular resistance.
Other Tests
Amplitude-integrated electroencephalography (aEEG)
Several studies have shown that a single-channel aEEG performed within a few hours of birth can help evaluate the severity of brain injury in the infant with hypoxic-ischemic encephalopathy (HIE). The abnormalities seen in infants with moderate-to-severe HIE include the following:
- Discontinuous tracing characterized by a lower margin below 5 mV and an upper margin above 10 mV
- Burst suppression pattern characterized by a background with minimum amplitude (0-2 mV) without variability and occasional high voltage bursts (>25 mV)
- Continuous low voltage pattern characterized by a continuous low voltage background (< 5 mV)
- Inactive pattern with no detectable cortical activity
- Seizures, usually seen as an abrupt rise in both the lower and upper margin
In addition, aEEG findings have been used as criteria for inclusion in the CoolCap trial of selective head cooling.However, some evidence argues against the use of aEEG as a tool to exclude infants with HIE from receiving hypothermia therapy.
Although normal aEEG findings may not necessarily mean that the brain is healthy, a severe or moderately severe aEEG abnormality may indicate brain injury and poor outcome. However, a rapid recovery (within 24 h) of abnormal aEEG findings is associated with favorable outcome in 60% of cases. Finally, in a meta-analysis of 8 studies, Spitzmiller et al concluded that aEEG can accurately predict poor outcome with a sensitivity of 91% and a negative likelihood ratio of 0.09
Note that considerable training is required for conducting and properly interpreting the aEEG findings. With more recent advancement in technology, aEEG can alert the caregivers regarding seizure activity based on the software recognition of a pattern.
Standard EEG
Traditional multichannel EEG is an integral part of the evaluation of infants diagnosed with HIE. It is a valuable tool to assess the severity of the injury and evaluate for electrographic-only seizures (which are very common in neonates with HIE). This is particularly important for infants on assisted ventilation requiring sedation or paralysis. EEG can be very useful to help a clinician decide treatment options based on the report’s findings.
Changes in EEG wave patterns evolve over time and are a reliable early indicator of the brain injury.
Generalized depression of the background rhythm and voltage, with varying degrees of superimposed seizures, are early findings. EEG characteristics associated with abnormal outcomes include (1) background amplitude of less than 30 mV, (2) interburst interval of more than 30 seconds, (3) electrographic seizures, and (4) absence of sleep-wake cycle at 48 hours.
Caution in interpreting early severe background abnormalities needs to be applied because reverting to normal background pattern in few days of life can be associated with normal outcomes. Note that large doses of anticonvulsant therapy may alter the EEG findings.
Serial EEGs should be obtained to assess seizure control and evolution of background abnormalities. Early EEGs are important not only to evaluate the degree of encephalopathy and the presence of seizures but may also help establish early prognosis. Serial EEGs are also helpful in establishing prognosis. Improvement in the EEG findings over the first week, in conjunction with improvement in the clinical condition, may help predict a better long-term outcome.
Special sensory evaluation
Screening for hearing is now mandatory in many states in the United States; in infants with hypoxic-ischemic encephalopathy, a full-scale hearing test is preferable because of an increased incidence of deafness among infants with hypoxic-ischemic encephalopathy that require assisted ventilation.
Retinal and ophthalmic examination
This examination may be valuable, particularly as part of an evaluation for developmental abnormalities of the brain.
Spectral-domain optical coherence tomography (SD-OCT) shows promise in the evaluation of prematurity on early optic nerve development and of central nervous system development and anomalies.
Important considerations
Birth asphyxia, birth injury, and perinatal asphyxia are terms often used incorrectly to describe hypoxic-ischemic encephalopathy (HIE).
A birth injury is a condition in which fetal or neonatal injury has occurred during the process of birth (ie, during the first and second stages of labor). Examples include brachial plexus injury; fracture of the clavicle; forceps-induced damage to the facial nerve or soft tissues; and cuts or bruises from scissors, clips, or scalp monitors.
Birth asphyxia is similar to birth injury in that asphyxia occurs during the first and second stages of labor when the fetus was otherwise normal.
Perinatal asphyxia signifies that asphyxia occurred around the time of delivery of a newborn baby.
The American Academy of Pediatrics (AAP) and American College of Obstetrics and Gynecology (ACOG) recommend using HIE because this term accurately describes the clinical condition, encephalopathy from asphyxia, without implying the time of brain injury. The AAP and ACOG also advise not using the terms perinatal asphyxia or birth asphyxia because it is difficult to identify the time of brain injury and nearly impossible to ascertain that the brain had been “normal” before such injury.
All professional societies encourage accurate recording of objective information in the medical records, including maternal and neonatal history and the clinical and laboratory findings.
The findings from brain imaging procedures and EEG help in the total assessment of the infant’s clinical status.
No diagnostic tests conclusively prove that a given magnitude of asphyxia has led to a specific neurologic injury. Acute perinatal and intrapartum events have been found only in about 20% of children diagnosed as having cerebral palsy.
Counseling the parents with available information and explanations about their infant’s clinical status and the prognosis is always recommended.
Diagnostic Considerations
Guidelines from the American Academy of Pediatrics (AAP) and the American College of Obstetrics and Gynecology (ACOG) for HIE indicate that all of the following must be present for the designation of perinatal asphyxia severe enough to result in acute neurologic injury:
- Profound metabolic or mixed acidemia (pH < 7) in an umbilical artery blood sample, if obtained
- Persistence of an Apgar score of 0-3 for longer than 5 minutes
- Neonatal neurologic sequelae (eg, seizures, coma, hypotonia)
- Multiple organ involvements (eg, kidney, lungs, liver, heart, intestines)
Other problems to be considered
Several inborn errors of metabolism can present in the neonatal period (usually not present at birth) with features similar to HIE. Those include the following:
- Nonketotic hyperglycinemia
- Disorders of pyruvate metabolism
- Urea cycle defects
- Zellweger syndrome
- Mitochondrial disorders
Other diagnoses should also be included in the differential diagnosis, including the following:
- Neuromuscular disorders including neonatal myopathies
- Brain tumors
- Developmental defects
- Infections
- Sulphite oxidase deficiency
Differential Diagnoses
- Genetics of Methylmalonic Acidemia
- Genetics of Propionic Acidemia (Propionyl CoA Carboxylase Deficiency)
Management
Following initial resuscitation and stabilization, treatment of HIE is largely supportive and should focus on the following:
- Adequate ventilation
- Perfusion and blood pressure management – Studies indicate that a mean blood pressure (BP) above 35-40 mm Hg is necessary to avoid decreased cerebral perfusion
- Careful fluid management
- Avoidance of hypoglycemia and hyperglycemia
- Avoidance of hyperthermia – Hyperthermia has been shown to be associated with increased risk of adverse outcomes in neonates with moderate to severe hypoxic-ischemic encephalopathy
- Treatment of seizures
- Therapeutic hypothermia (33º-33.5ºC for 72h) followed by slow and controlled rewarming for infants with moderate to severe HIE
Initial Resuscitation and Stabilization
Delivery room management follows standard Neonatal Resuscitation Program (NRP) guidelines. Close attention should be paid to appropriate oxygen delivery, perfusion status, avoidance of hypoglycemia and hyperglycemia, as well as avoidance of hyperthermia.
A lot of attention has been focused on resuscitation with room air versus 100% oxygen in the delivery room. Several clinical trials indicate that room air resuscitation for infants with perinatal asphyxia is as effective as resuscitation with 100% oxygen. In addition, infants resuscitated with room air have a lower level of circulating markers of oxidative stress. However, studies indicating that time to return to spontaneous circulation is equivalent with room air resuscitation are lacking. Based on this evidence, International Liaison Committee on Resuscitation (ILCOR) and NRP guidelines were updated and are now recommending the use of 21% oxygen for the initial resuscitation of term infants. If despite effective ventilation, the infant does not improve, higher concentrations of oxygen should be used and should be guided by the use of pulse oxymetry.
Supportive Care in Patients with Hypoxic-ischemic Encephalopathy
Most infants with severe hypoxic-ischemic encephalopathy (HIE) need ventilatory support during the first few days after birth. Although animal data suggest that permissive hypercapnia may be neuroprotective, no such evidence is available in newborn. Therefore, the role of mechanical ventilation is to maintain the blood gases and acid-base status in the physiologic ranges and prevent hypoxia, hyperoxia, hypercapnia, and hypocapnia. Hypocapnia in particular may lead to severe brain hypoperfusion and cellular alkalosis and has been associated with worse neurodevelopmental outcomes. Of note, evidence indicates that increased FiO2 in the first 6 hours of life is a significant risk factor for adverse outcomes in infants with hypoxic-ischemic encephalopathy treated with hypothermia therapy. This association is independent of underlying respiratory pathology and further emphasizes the benefit of resuscitation and stabilization with room air in this patient population.
Infants with HIE are also at risk for pulmonary hypertension and should be monitored. Inhaled nitric oxide (iNO) may be used according to published guidelines if pulmonary hypertension is suspected.
Perfusion and Blood Pressure Management
Studies indicate that a mean blood pressure (BP) above 35-40 mm Hg is necessary to avoid decreased cerebral perfusion. Hypotension is common in infants with severe hypoxic-ischemic encephalopathy (HIE) and is due to myocardial dysfunction, capillary leak syndrome, and hypovolemia; hypotension should be promptly treated. Dopamine or dobutamine can be used to achieve adequate cardiac output in these patients. If a cardiac injury is suspected, then administration of dobutamine or milrinone may be beneficial to support the injured heart.
Fluid and Electrolytes Management
Because of the concern for acute tubular necrosis (ATN) and syndrome of inappropriate antidiuretic hormone (SIADH) secretion, fluid restriction is typically recommended for these infants until renal function and urine output can be evaluated. However, this recommendation is not based on evidence from randomized controlled trials. Therefore, fluid and electrolyte management must be individualized on the basis of clinical course, changes in weight, urine output, and the results of serum electrolyte and renal function studies.
The role of prophylactic theophylline, given early after birth, in reducing renal dysfunction after hypoxic-ischemic encephalopathy (HIE) has been evaluated in 3 small randomized controlled trials. In these studies, a single dose of theophylline (5-8 mg/kg) given within 1 hour of birth resulted in (1) decreased severe renal dysfunction (defined as creatinine level >1.5 mg/dL for 2 consecutive days); (2) increased creatine clearance; (3) increased glomerular filtration rate (GFR); and (4) decreased b2 microglobulin excretion. The clinical significance of these findings remains unclear. Larger studies are warranted to confirm the safety of adenosine inhibitor use following HIE.
Fluid and glucose homeostasis should be achieved. Avoid hypoglycemia and hyperglycemia because both may accentuate brain damage. Hypoglycemia in particular should be avoided. In a retrospective study, Salhab et al showed that initial hypoglycemia (< 40 mg/dL) is significantly associated with adverse neurologic outsomes.
Hyperthermia Avoidance
Hyperthermia has been shown to be associated with increased risk of adverse outcomes in neonates with moderate-to-severe hypoxic-ischemic encephalopathy (HIE).In this observational secondary study, the risk of death or moderate-to-severe disability was increased 3.6-fold to 4-fold for every 1°C increase in the mean of the highest quartile of skin or esophageal temperature.
Treatment of Seizures
Hypoxic-ischemic encephalopathy (HIE) is the most common cause of seizures in the neonatal period. Seizures are generally self-limited to the first days after birth but may significantly compromise other body functions, such as maintenance of ventilation, oxygenation, and blood pressure. Additionally, studies suggest that seizures, including asymptomatic electrographic seizures, may contribute to brain injury and increase the risk of subsequent epilepsy.
Current therapies available to treat neonates with seizures have limited efficacy, and safety concerns remain specifically for infants undergoing therapeutic hypothermia. Antiseizure drugs used in this population include phenobarbital, levetiracetam, phenytoin, lidocaine, and benzodiazepines. However, phenobarbital has been shown to be effective in only 29-50% of cases, and phenytoin only offers an additional 15% efficacy. Benzodiazepines, particularly lorazepam, may offer some additional efficacy. Newer antiseizure medications such as levetiracetam are increasingly used in infants with HIE and seizures despite the lack of strong evidence regarding safety or efficacy in this population.
Hypothermia Therapy
Extensive experimental data suggest that mild hypothermia (3-4°C below baseline temperature) applied within a few hours (no later than 6 h) of injury is neuroprotective. The neuroprotective mechanisms are not completely understood. Possible mechanisms include (1) reduced metabolic rate and energy depletion; (2) decreased excitatory transmitter release; (3) reduced alterations in ion flux; (4) reduced apoptosis due to hypoxic-ischemic encephalopathy; and (4) reduced vascular permeability, edema, and disruptions of blood-brain barrier functions.
Following initial resuscitation and stabilization, treatment of HIE includes hypothermia therapy for moderate to severe encephalopathy as well as supportive measures focusing on adequate oxygenation, ventilation and perfusion, careful fluid management, avoidance of hypoglycemia and hyperglycemia, and treatment of seizures. Intervention strategies aim to avoid any further brain injury in these infants.
In patients with HIE and suspected neonatal sepsis receiving gentamicin and hypothermia treatment, modified gentamicin dosing regimens are required owing to the reduced clearance of this agent potentially leading to toxicity in these infants from higher gentamicin concentrations during hypothermia therapy.
Adverse effects
Many theoretical concerns surround hypothermia and its side effects, which include coagulation defects, leukocyte malfunctions, pulmonary hypertension, worsening of metabolic acidosis, and abnormalities of cardiac rhythm, especially during rewarming.
Randomized trials have been reassuring thus far regarding the safety and applicability of therapeutic hypothermia. In a 2013 Cochrane review, significant adverse effects were limited to sinus bradycardia and thrombocytopenia.
Transfer
Infants who present in a level I or II center may require transfer to a tertiary neonatal intensive care unit (NICU) for definitive neurodiagnostic studies (electroencephalography [EEG] and neuroimaging), consultation with a pediatric neurologist, and evaluation for therapeutic hypothermia. Based on the current recommendations, therapeutic hypothermia must be initiated within 6 hours after birth. Timely referral is essential to provide therapeutic hypothermia. If that window (of 6 hours) has passed, infants will still benefit from the expertise of level III and higher centers.
Discharge considerations
Physical therapy and developmental evaluations are needed prior to discharge of patients with HIE. Even after discharge, close monitoring and regular follow-ups are essential for better outcomes. Referring to early intervention is a must at the time of discharge.
Continuation of seizure medications should depend on evolving central nervous system (CNS) symptoms and EEG findings. In most cases, antiseizure medications can be discontinued prior to NICU discharge. Follow-up by a pediatric neurologist is recommended.
Prognosis
Accurate prediction of the severity of long-term complications of hypoxic-ischemic encephalopathy (HIE) is difficult, although clinical, laboratory, and imaging criteria have been used. The following criteria have been shown to be the most helpful in outlining likely outcomes:
- Lack of spontaneous respiratory effort within 20-30 minutes of birth is almost always associated with death.
- The presence of seizures is an ominous sign. The risk of poor neurologic outcome is distinctly greater in such infants, particularly if seizures occur frequently and are difficult to control.
- Abnormal clinical neurologic findings persisting beyond the first 7-10 days of life usually indicate poor prognosis. Among these, abnormalities of muscle tone and posture (hypotonia, rigidity, weakness) should be carefully noted.
- EEG at about 7 days that reveals normal background activity is a good prognostic sign.
- Persistent feeding difficulties, which generally are due to abnormal tone of the muscles of sucking and swallowing, also suggest significant CNS damage.
- Poor head growth during the postnatal period and the first year of life is a sensitive finding predicting higher frequency of neurologic deficits.
A Swedish retrospective population-based study comprising 692,428 live births of at least 36 gestational weeks found that more than a quarter (29%) of all HIE births were associated with an obstetric emergency, with parous women affected more than nulliparous women. The investigators noted a strong association of shoulder dystocia in nulliparas, and to uterine rupture in women with previous cesarean deliveries.
Morbidity/mortality
In severe HIE, the mortality rate is reportedly 25-50%. Most deaths occur in the first days after birth due to multiple organ failure or redirection of care to comfort measures as a result of the grim prognosis. Some infants with severe neurologic disabilities die in their infancy from aspiration pneumonia or systemic infections.
The incidence of long-term complications depends on the severity of HIE. As many as 80% of infants who survive severe HIE develop serious complications, 10-20% develop moderately serious disabilities, and as many as 10% are healthy. Among the infants who survive moderately severe HIE, 30-50% may have serious long-term complications, and 10-20% have minor neurologic morbidities. Infants with mild HIE tend to be free from serious CNS complications.
Two therapeutic hypothermia trials provided updated information on mortality and the incidence of abnormal neurodevelopmental outcomes in infants with moderate to severe HIE. In these trials, 23-27% of infants died prior to discharge from the neonatal intensive care unit (NICU), whereas the mortality rate at follow-up 18-22 months later was 37-38%. In these trials, neurodevelopmental outcomes at 18 months were as follows:
- Mental development index (MDI): Scores of 85 or higher, 40%; 70-84, 21%; less than 70, 39%
- Psychomotor development index (PDI): Scores of 85 or higher, 55%; 70-84, 10%; less than 70, 35-41%
- Disabling cerebral palsy – 30%
- Epilepsy – 16%
- Blindness – 14-17%
- Severe hearing impairment – 6%
Data from a randomized controlled trial was evaluated to determine the relationship between hypocarbia and the outcome for neonatal patients with hypoxic-ischemic encephalopathy. The results found that a poor outcome (death/disability at 18-22 months) was associated with a minimum partial pressure of carbon dioxide (PCO2) and cumulative PCO2 of less than 35 mm Hg; death and disability increased with greater exposure to PCO2 of less than 35 mm Hg.
Even in the absence of obvious neurologic deficits in the newborn period, long-term functional impairments may be present. In a cohort of school-aged children with a history of moderately severe HIE, 15-20% had significant learning difficulties, even in the absence of obvious signs of brain injury. Thus, all children who have moderate or severe HIE should be monitored well into school age.
Consultations
A pediatric neurologist should help assist in the management of seizures, interpretation of electroencephalograms (EEGs), and overall care of the infant with hypoxic-ischemic encephalopathy (HIE). The neurologist should also work with the primary care physician to address long-term disabilities.
Follow-up by a developmental pediatrician is also recommended to assist with planning for the infant’s long-term assessments of neurodevelopment and care.
Diet
In most cases (particularly in severe hypoxic-ischemic encephalopathy [HIE]), the infant is restricted to nothing by mouth (NPO) until the general level of alertness and consciousness improves and the hemodynamic status stabilizes. In addition, most infants undergoing therapeutic hypothermia should remain NPO until rewarmed. A study of 51 neonates with HIE indicated that minimal enteral nutrition (1-2 mL/kg boluses every 3h) may be safe in hemodynamically stable infants undergoing therapeutic hypothermia.
Enteral feeds should be carefully initiated, and the use of trophic feeds is recommended for 24-48 hours (2 mL/kg every 3 h). Infants should be monitored carefully for signs and symptoms of necrotizing enterocolitis, for which infants with perinatal asphyxia are at high risk. Individualize increments in feeding volume and composition.
Prevention
The use of intrapartum markers, such as fetal heart rate monitoring, are poor predictors of neonatal outcomes and long-term risk of cerebral palsy.
Most treatments under investigation have been discussed earlier and remain experimental. Except for therapeutic hypothermia, no treatment has consistently shown efficacy in human infants.
Patient Education
Parents are often concerned about infants’ pain and distress, parental-infant bonding, and outcomes following hypothermia treatment. Keys to reassuring parents of infants undergoing hyperthermia, include consistent communication, regular updates, and early, balanced discussions regarding potential long-term outcomes; parental involvement in decision making; and having strong support mechanisms.
Long-Term Monitoring
The goal of follow-up is to detect impairments and promote early intervention for those infants who require it.
Growth parameters including head circumference should be closely monitored in all infants with hypoxic-ischemic encephalopathy (HIE).
Infants with moderate-to-severe HIE should be followed closely after neonatal intensive care unit (NICU) discharge by a developmental pediatrician and, in some cases, a pediatric neurologist (if there is a history of seizure and/or abnormal neurologic examination). Additionally, evaluation by a pediatric ophthalmologist is recommended during the first year of life, because damage to the posterovisual cortex can occur. Standard hearing test screening should occur prior to NICU discharge. A repeat hearing screen is also recommended in the first 2 years of life.
If therapeutic hypothermia was used in the neonatal period, follow-up is recommended for the continued evaluation of the long-term efficacy of this therapy. Data should be entered into the available registries, local databases, or both, whenever possible.
Infants with mild HIE generally do well and do not require specialized follow-up.
Future Neuroprotective Strategies
Several groups are investigating other neuroprotective strategies whether alone or in combination with hypothermia therapy (summarized in the image below).
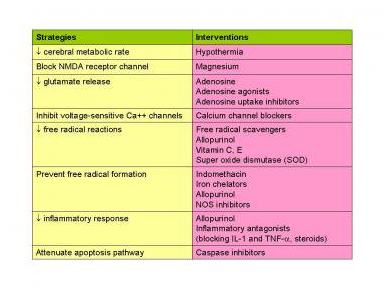
Summary of potential neuroprotective strategies.
Promising avenues include the following:
- Prophylactic barbiturates: In a small randomized trial, high-dose phenobarbital (40 mg/kg) was given over 1 hour to infants with severe hypoxic-ischemic encephalopathy. Treated infants had fewer seizures (9 of 15) than untreated control infants (14 of 16). Treated infants also had fewer neurologic deficits at age 3 years (4 of 15) than untreated infants (13 of 16).In another small study, thiopental given within 2 hours and over 24 hours, did not result in improved rate of seizures or neurodevelopmental outcomes at 12 months. Hypotension was more common in infants who received thiopental. Thus, the role of prophylactic barbiturate remains unclear. Further studies are needed.
- Erythropoietin: In one study, low-dose erythropoietin (300-500 U/kg) administered for 2 weeks starting in the first 48 hours of life decreased the incidence of death or moderate and severe disability at age 18 months (43.8% vs 24.6%; P < 0.05) in infants with moderate-to-severe hypoxic-ischemic encephalopathy. Subgroup analysis indicated that only infants with moderate disability benefited from this therapy.
- Allopurinol: Slight improvements in survival and cerebral blood flow (CBF) were noted in a small group of infants tested with this free-radical scavenger in one clinical trial.
- Excitatory amino acid (EAA) antagonists: MK-801, an EAA antagonist, has shown promising results in experimental animals and in a limited number of adult trials. However, this drug has serious cardiovascular adverse effects.
- Stem cell therapy: The use of mesenchymal stem cells and autologous stem cells to treat infants with hypoxic-ischemic encephalopathy (HIE) is under extensive study. Early evidence suggests this may be an effective therapeutic avenue. More work is required to determine the type of cells, dose, timing, and duration. In addition, more studies are also required to understand the underlying protective mechanisms.
- Other adjuvant therapies under investigation include Xexon (NCT0271394 and NCT01545271), topiramate (NCT01765218), and MgSO4 (NCT01646619).

Leave a Reply
Want to join the discussion?Feel free to contribute!