Spinal Epidural Abscess: Diagnosis, Management, and Outcomes
Abstract
An infection of the spinal epidural space, spinal epidural abscess (SEA) is a potentially devastating entity that is rising in incidence. Its insidious presentation, variable progression, and potential for precipitous neurologic decline make diagnosis and management of SEA challenging. Prompt diagnosis is key because treatment delay can lead to paralysis or death. Owing to the nonspecific symptoms and signs of SEA, misdiagnosis is alarmingly common. Risk factor assessment to determine the need for definitive MRI reduces diagnostic delays compared with relying on clinical or laboratory findings alone. Although decompression has long been considered the benchmark for SEA, the considerable risk associated with spinal surgery is noted in an older cohort with multiple comorbidities. Nonoperative management may represent an alternative in select cases. Failure of nonoperative management is a feared outcome associated with motor deterioration and poor clinical outcomes. Recent studies have identified independent predictors of failure and residual neurologic dysfunction, recurrence, and mortality. Importantly, these studies provide tools that generate probabilities of these outcomes.
Introduction
Spinal epidural abscess (SEA) is a rare and challenging entity that represents a suppurative process in the spinal epidural space. Its low incidence, insidious presentation, and precipitous neurologic decline make diagnosis and management of SEA challenging. The increasing incidence of SEA may be due to higher rates of risk factors for SEA in the modern cohort: increased age, immunosuppressive comorbidities (e.g., diabetes mellitus, end-stage renal disease, and malignancy), intravenous drug use, and spinal procedures.
Pathogenesis
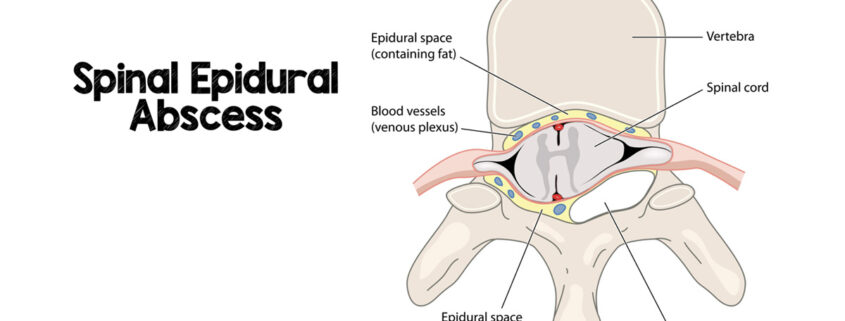
The spinal epidural space is a potential space between the dura mater and vertebral bone that contains fatty tissue and a rich venous plexus. The venous drainage of the spinal column and epidural space communicates with the systemic circulation via the Batson plexus; this valveless venous network has a bidirectional flow that allows for the potential spread of infection from local or distant foci. The epidural space may become infected through either hematogenous dissemination or contiguous spread from local infections such as paraspinal abscess, osteomyelitis, and/or discitis. Although bacterial and fungal infections of the epidural space have been reported, bacterial SEA is by far the most common. Expanding purulence within the epidural space results in pain, systemic symptoms, and eventually spinal cord or nerve root dysfunction.
Considerable debate exists over the pathophysiology underlying spinal cord injury in SEA. Theories regarding the mechanism of spinal cord dysfunction include the following: mechanical compression by the abscess, thrombosis of major spinal vessels, impairment of intrinsic circulation of the cord, and abscess-induced vasculitis.
Mechanical compression of the spinal cord and nerve roots by an expanding abscess has been posited to be a major contributor to neurologic deficits in SEA. Histopathological analyses of rabbit models of SEA by Feldenzer et al suggested that mechanical injury is more responsible for spinal cord dysfunction than ischemic injury. The combination of progressive compression and ischemia may be responsible for the severity and finality of neurologic compromise in advanced SEA. Multiple studies have reported a correlation between the increasing degree of spinal cord compression and motor deficit at presentation. The observation that decompression often leads to reversal of deficits supports this theory.
Although the increasing mass effect is associated with neurologic dysfunction at presentation, destructive changes in the spinal cord in SEA is not completely explained by mechanical compression alone. Postmortem studies of patients with SEA have often shown infarction of the cord without cord deformity. Furthermore, inflammatory processes such as leukocytosis are associated with motor deficits in SEA.
It is likely that a combination of compressive and vascular etiologies is responsible for neurologic dysfunction in SEA. An initial neurologic deficit may be due to mechanical compression of the spinal cord, whereas residual motor deficit may be due to inflammation-induced infarction.
Clinical Features
Heusner described four progressive phases of the disease: spinal ache, root pain, weakness, and paralysis. The duration of each stage is variable, and precipitous decline in neurologic status may occur unpredictably, underlining the importance of prompt diagnosis and treatment.
Diagnosis of SEA has long been defined by the presence of a classic clinical triad of the focal spine back, fever, and neurologic deficit. The presence of the triad is not reliable, however, because it is inconsistently observed at presentation. The most common symptoms of SEA include back pain, radicular pain, motor or sensory dysfunction, fever, and bladder/bowel dysfunction. Back pain is present in most patients with SEA and is often the initial report. Incomplete or complete cord injury is present in 35% to 50% of patients at presentation. The motor deficit in SEA is believed to be due to a combination of mechanical compression of the spinal cord and/or nerve roots and ischemia from vascular compromise. In a study of 1,053 patients with SEA, Shah et al reported that an abscess located cephalad to the conus medullaris is significantly associated with the motor deficit at presentation. It has been hypothesized that the less abundant epidural space in the cervical and thoracic spine may allow a smaller abscess to injure the spinal cord with resulting neurologic injury. By contrast, only nerve roots are present in the relatively abundant lumbar epidural space below the conus, thereby limiting cord injury from abscesses in this region.
Earlier reports show a predilection for the thoracic spine due perhaps to the larger thoracolumbar epidural space. Recent studies report that the lumbar spine is the most common location of SEA; this is likely because of an increased prevalence of spinal procedures, the bulk of which involve the lumbar spine. Abscesses span three to four vertebral levels on average. Patients are commonly bacteremic at presentation, 58% of patients in the largest series. Staphyloccocus aureus is the most commonly responsible organism, with methicillin-sensitive S aureus more often identified than methicillin-resistant strains.
Diagnosis
Prompt diagnosis and treatment of SEA is key because delay can lead to paralysis or death. Accurate diagnosis of SEA is difficult because of its low incidence and nonspecific presentation (e.g., back pain, fever, leukocytosis, and elevated inflammatory markers). These nonspecific symptoms may also keep patients from seeking care until the disease stage has progressed. Although abnormal laboratory values such as leukocytosis or elevated inflammatory markers may be predictive of disease severity in established disease, they are not specific for diagnosis.
Misdiagnosis is alarmingly common, with up to 75% of cases misdiagnosed. This is especially the case in patients who are neurologically intact at presentation. Afebrile patients without elevated inflammatory markers or leukocytosis are often misdiagnosed with more common causes of back pain such as degenerative joint disease or intervertebral disk herniation. Patients with a more infectious presentation may be misdiagnosed with more common pathology such as vertebral osteomyelitis, discitis, or urinary tract infection. Delay in diagnosis leads to a subsequent delay in initiation of treatment, allowing the infection to progress to a more advanced state. Unsurprisingly, a significantly higher incidence of the residual motor deficit is observed in patients with diagnostic delay.
Owing to the difficulty of diagnosis, the classic diagnostic triad is still relied on by many clinicians. Many SEA cases are thus not suspected or identified without the presence of neurologic deficit, delaying diagnosis, and treatment. The low sensitivity of the triad and its reliance on the presence of neurologic dysfunction limits its clinical utility in the diagnostic setting. In a consecutive series of 74 patients with SEA, Davis et al found that risk factor screening is far more sensitive than the classic triad. They followed up their retrospective analysis with a prospective study in 2011 in which they show a significant reduction in diagnostic delays by implementing a novel decision guideline using risk factor assessment followed by ESR and C-RP testing before obtaining definitive imaging for suspected SEA. The risk factor assessment includes established risk factors for SEA such as diabetes, immunosuppression, chronic liver/kidney disease, recent spinal procedure, and intravenous drug use. Risk factor assessment focuses on the underlying pathophysiology of SEA, whereas the classic triad identifies sequelae of SEA. Because the seeding of the epidural space with bacteria is the initial insult that results in SEA, risk factors for bacteremia (e.g., intravenous drug use and indwelling catheters) or direct inoculation (e.g., indwelling spinal hardware and recent spinal procedures) should increase suspicion for SEA. A complete medical history and physical examination with a complete neurologic examination with documentation of motor and sensory function should be a mandatory part of the evaluation of any patient suspected of SEA.
Imaging and Classification
Lumbar puncture results demonstrating parameningeal inflammation has been previously described to aid diagnosis of SEA; however, this is no longer routinely done. Definitive diagnosis of SEA is best achieved with gadolinium-enhanced MRI, with a sensitivity and specificity greater than 90%. It is non-invasive and allows for delineation of abscess extension in the longitudinal plane. The abscess appears iso- or hypo-intense to the spinal cord on T1-weighted MRI and hyperintense to the spinal cord on T2-weighted images (Figure 1, A and B). If MRI cannot be obtained, CT with IV contrast is the next best choice. Although CT myelography is nearly as sensitive as MRI, it is more invasive and thus introduces the risk of spreading the infection to the subarachnoid space or causing meningitis. It is important to obtain imaging of the entire spine if there is a concern for skip lesions. Risk factors for the presence of skip lesions include delay in presentation, concomitant extraspinal infection, and elevated ESR.
Imaging reveals not only the presence and extent of SEA but also the degree of spinal cord compression. Although many studies have linked the increasing mass effect on the spinal cord with neurologic dysfunction at presentation, their generalizability is limited by the lack of a consistent definition of the degree of cord compression. A recent study proposed a grading system for cord compression in SEA based on axial MRI images—modified from a grading system of epidural spinal cord compression from metastatic spinal tumors—showing an association between the increasing degree of compression and motor deficit, sensory dysfunction, and bladder/bowel dysfunction.
Management
When diagnosis of SEA is suspected, prompt treatment is necessary. Empiric antibiotic treatment is started in nearly all patients and is narrowed if speciation is possible. Considerable debate exists regarding whether the appropriate initial treatment modality ought to be surgical versus nonsurgical in nature.
Surgical Management
Surgical decompression of the abscess has long been considered the benchmark for the management of SEA, particularly in the setting of neurologic dysfunction. Multiple studies have reported that early evacuation is associated with improvement or maintenance of preoperative neurologic status. Patients who present with the focal motor deficit are more likely to show neurologic improvement with surgical intervention.
Laminectomy alone has the advantage of lower cost and a relatively low-risk profile when compared with laminectomy plus fusion; however, it leaves patients susceptible to spinal instability, especially in the cervical spine. In the setting of a liquid abscess with free-flowing pus located dorsal to the thecal sac, laminotomy or hemilaminectomy may provide adequate decompression; this approach provides the advantage of leaving the paraspinal musculature and interspinous ligaments intact on the contralateral side. If a solid phlegmatic component exists or if more than two levels need to be decompressed, a more aggressive approach may be required with the need for instrumented fusion. If a laminectomy is done at the apex of kyphosis or at the thoracolumbar junction, instrumentation may be considered. In addition to the benefit of added stability, instrumented fusion may reduce the risk of postoperative kyphosis and allow for the early mobilization of patients. Although spinal instrumentation in the setting of active infection raises concern for continued infection, a recent study of 572 patients who underwent surgery for SEA showed that the use of instrumentation was not associated with recurrent SEA. The addition of fusion, however, is associated with higher rates of blood transfusion and reoperation compared with laminectomy alone; the benefit of added spinal stability must be weighed against these risks. In single-level or two-level cases where the risk of postoperative kyphosis is low, the reoperation risk of fusion may outweigh the benefit of avoiding future kyphosis; however, in multilevel procedures, the high rate of postoperative kyphosis development may tip the balance toward fusing.
The surgical management depends on the location of the abscess and the degree of involvement of the vertebral body. In the cervical spine, it is acceptable to proceed with posterior decompression with or without fusion if the involvement is primarily epidural without severe destruction of the vertebral body. If the anterior elements are affected by osteomyelitis and/or discitis, débridement with corpectomy with or without diskectomy and interbody fusion with possible anterior plate stabilization may be required. In the thoracic spine, anterior decompression and fusion with bone graft supplemented with posterior instrumentation may be required in the setting of vertebral body involvement or collapse. A combined anterior and posterior approach may be needed in the presence of anterior bony destruction or collapse in the lumbar spine.
Nonoperative Management
Although surgical management has historically been the preferred treatment modality for SEA, the risks of spinal surgery in a cohort more likely to be of advanced age and with multiple medical comorbidities are considerable. Nonoperative management has increased in popularity in recent decades. In a recent systematic review, Arko et al found that a significantly greater proportion of patients received medical management after 1999 than before it. Systemic antibiotic therapy with or without CT-guided drainage may be an acceptable alternative to surgery in selected cases. Nonoperative management may be appropriate in patients with intact neurologic status, multiple medical comorbidities that preclude safe surgery, panspinal involvement, or paralysis for >48 hours. In a recent systematic review, the major factors determining whether a patient received surgery were the presence of motor weakness or other neurologic symptoms. Medical management may also be indicated in pediatric patients because of the concern for post-laminectomy kyphosis.
With a cohort of 48 patients with SEA, Curry et al reported that outcomes are significantly worse in patients treated conservatively versus surgically. Apart from this study, there have been few studies that found a difference in outcomes between medical and surgical management for SEA. Some authors have advocated for surgical decompression, some have advocated for nonoperative management, and still others have stated that no difference was observed between the two modalities. With the data currently available in the literature, it is not possible to determine whether one treatment strategy is superior to the other; rather, it is more prudent to determine in which setting it is more appropriate to use one over the other.
Failure of Nonoperative Management
Nonoperative management of SEA is not without its own set of risks; failure of nonoperative management carries a dire prognosis. Patients who fail medical management have been shown to have significant deterioration of their motor status. The ability to recover motor function after treatment failure is impaired, particularly with abscesses located in the cervical spine. Furthermore, treatment failure may also result in sepsis, bone loss, and possible development of bacterial resistance in the setting of protracted antibiosis. It is thus imperative to avoid failure of the nonoperative management of SEA. Until recently, there have been limited data with small cohort sizes in the literature regarding the rate of failure or risk factors for nonoperative management failure—generally defined as neurologic deterioration, persistent or worsening symptoms, or progression on serial imaging despite initiation of systemic antibiotic therapy. Recent studies on failure of nonoperative management have reported failure rates ranging from 27% to 41%.
One of the first studies to identify independent predictors of nonoperative management failure was published by Kim et al in 2014. With a cohort of 142 medically managed patients, they identified four independent predictors of failure: age >65 years, diabetes, methicillin-resistant S aureus, and neurologic impairment. If all four risk factors are present, they report a 99% failure rate. Patel et al, 2014 conducted a similar study, also reporting four independent predictors of failure: diabetes, leukocytosis greater than 12.5, positive blood cultures, and C-reactive protein >115. If three or more of these are present, a 77% risk of failure exists. Most recently, Shah et al identified six independent predictors of failure using a cohort of 367 medically managed patients: motor deficit at presentation, pathologic/compression fracture in affected levels, active malignancy, diabetes, sensory deficit, and/or paresthesia. An exclusively dorsal abscess was found to be relatively protective from failure when compared with abscesses with a ventral component.
Both Kim et al and Shah et al identified pretreatment motor deficit as a risk factor for failure; all three of the studies identified diabetes as an independent predictor.
Pretreatment motor deficit is an established risk factor for failure of nonoperative management, as is another neurologic dysfunction such as sensory deficit or paresthesias. This finding is in line with the long-standing observation that neurologic deficit at the time of presentation is associated with poor long-term prognosis. Diabetes is associated with impaired immune response and diminished integrity of the spinal microvasculature. Interestingly, there have been conflicting reports regarding the association between the anatomy of the abscess relative to the thecal sac and failure of nonoperative management. Karikari et al. concluded that ventral SEA is more likely to be treated successfully with conservative management than dorsal abscesses. They postulate that because ventral SEA occurs from the seeding of the intervertebral disk space or epidural fat with extension into the epidural space, these abscesses are more likely to present with systemic symptoms before neurologic deficits, thereby seeking medical attention earlier in the disease course. By contrast, Shah et al reported that an exclusively dorsal abscess is relatively protective against failure of nonoperative management. This may be due to a lower risk of disruption of the anterior spinal artery—the dominant spinal cord supply vessel—with a dorsal abscess; compression of this critical vessel may cause cord ischemia and worsened disease.
Given the neurologic risk incurred by the failure of nonoperative management, the use of algorithms to gauge the risk of failure is of great utility. The studies by Kim, Patel, and Shah each offer tools for the prediction of nonoperative management failure ranging from tables offering probability of failure based on the number of risk factors to an algorithm that generates the risk of failure based on points assigned to the presence or absence of risk factors. More recently, machine learning algorithms have been developed to successfully predict nonoperative management failure. Building off their previous multivariable logistic regression algorithm, Shah et al reported a discriminative and well-calibrated model that is incorporated into a web-based application that is designed to inform clinical decision-making in real-time.
Outcomes
In Heusner’s original series of 20 patients, 45% of patients either died or had residual paralysis. Although outcomes have improved since then, SEA remains a disease with significant morbidity and mortality.
Residual Neurologic Deficit
Prompt diagnosis and treatment of SEA is important because of the unpredictable and often rapid decline in neurologic function, with irreversible paralysis being a feared sequela of inadequately treated SEA. The single most important predictor of residual neurologic outcome is the patient’s neurologic status before intervention. More severe preoperative neurologic deficits are associated with worse outcomes. Early diagnosis of SEA is crucial; patients accurately diagnosed with SEA at presentation are less likely to deteriorate neurologically with subsequent residual neurologic deficits. Patients operated on earlier in their disease course tend to have better outcomes. Nonoperatively managed patients on average had lower motor scores compared with pretreatment. This is primarily because of the significant deterioration in motor scores in patients who failed nonoperative management. An increasing degree of cord compression as defined by the classification system proposed by Shah et al. is associated with pretreatment motor deficit; interestingly, it is not associated with post-treatment motor deficit.
Recurrence
Sequelae of an inadequately treated SEA that recurs are often dire. Data regarding the rate and risk factors of recurrence have remained scarce in the literature. With a cohort of 27 patients with SEA managed surgically, Lohr et al reported a recurrence rate of 30%. They identify that the longitudinal extent of the abscess and the presence of granulation tissue in the epidural space are predictive of recurrence. More recently, Shah et al studied a series of 572 patients with SEA treated surgically or nonsurgically and found that 6.6% had documented recurrence with a median time to recurrence of 20 weeks postadmission. Furthermore, they identify three independent predictors of recurrence: history of intravenous drug use, bowel dysfunction, and local surgical site infection after spinal surgery. An important risk factor for the development of SEA and intravenous drug use is associated with diminished humoral and cellular immunity. Furthermore, the reluctance of an inpatient team to discharge a patient with a history of intravenous drug use with a peripherally inserted central catheter for an extended outpatient course of intravenous antibiotics may result in the discharge of patients with an inadequate oral antibiotic course. The presence of bowel dysfunction at presentation suggests an advanced stage of disease with spinal cord injury; successful eradication of an abscess this far along in the disease process is challenging. A concurrent local wound infection complicates comprehensive source control. It is important that all patients treated for SEA are closely followed; this is of particular importance in patients with risk factors for disease recurrence.
Mortality
Before systemic antibiotic therapy was widespread, spinal infections were often life-threatening with a mortality rate reaching 70%. Mortality in SEA has decreased significantly, with reported rates ranging from 1% and 16%. Multiple recent studies have identified independent predictors of 30-day and 90-day mortalities. Like those of nonoperative management failure, these studies use both multivariable logistic regression and machine learning methods.
Advanced age greater than 60 or 65 years is associated with increased risk of mortality. Patients who are underinsured are more likely than privately insured patients to have in-hospital mortality. Medical comorbidities that are associated with chronic disease states such as end-stage renal disease, active malignancy, and diabetes are predictors of mortality. Patients with SEA usually die because of uncontrolled sepsis or exacerbation of other underlying illnesses. Leukocytosis, thrombocytopenia, hypoalbuminemia, and neutrophil to lymphocyte ratio—laboratory abnormalities commonly seen in a systemic inflammatory state—have also been linked to mortality in SEA. Indicative of a systemic infection, the presence of endocarditis has been found to be associated with 90-day mortality. Furthermore, the following perioperative complications are associated with 30-day mortality: septic shock, cardiac arrest, pneumonia, and multiple blood transfusions. Finally, the presence of pretreatment motor deficit has been repeatedly demonstrated to be predictive of mortality.
Most of the aforementioned risk factors represent chronic disease states or patient characteristics that are nonmodifiable. The one significant exception to this is pretreatment motor deficit, which represents a potentially modifiable risk factor. Early diagnosis and treatment before the onset of motor deficit may lead to a reduction in mortality, again emphasizing the importance of having a low index of suspicion for diagnosis and prompt management of SEA.
Medicolegal Considerations
It is an unavoidable fact that the modern practice of medicine in the United States is influenced in varying degrees by medicolegal considerations. The diagnostic challenge and high risk of neurologic deterioration and mortality encountered in SEA elevates the risk of malpractice litigation. The clinician responsible for making the initial diagnosis (e.g., internist and emergency medicine physician) was most likely to be named as a defendant. Neurologic deterioration, delayed diagnosis, and delayed treatment are all factors predictive of a ruling in favor of the plaintiff. Delay in treatment was alleged when plaintiffs argue that surgery was delayed despite a known diagnosis of SEA. This consideration may be a motivating factor for providers to opt for surgical management even in situations where nonoperative management may be successful. This reality underlines the importance of educating our internal medicine and emergency medicine colleagues on the inclusion of SEA in the differential diagnosis to facilitate early diagnosis. Early involvement of orthopaedic and neurological surgeons in the management of any suspected spinal infection is also key. Ultimately, the decision to treat is between an individual clinician and his/her patient, based on the level of risk they are willing to assume. Acceptable risk thresholds vary from physician to physician and from patient to patient; accurate predictive models help frame the risk of failure of nonoperative management or mortality.
Conclusions
SEA is a challenging disease to diagnose and manage, requiring the expertise and insight of multiple medical and surgical disciplines (e.g., emergency medicine, internal medicine, radiology, neurology, orthopedic surgery, and neurosurgery). Although many questions regarding the pathophysiology and management of SEA remain inadequately answered, much work has been conducted recently to further understand this dangerous entity and provide guidance for clinical decision-making. Multiple studies have attempted to use predictive modeling for outcomes of interest in SEA with varying degrees of success. Reliable predictive models require a robust sample size with adequate statistical power; this is often difficult to obtain for a disease process with such a low incidence.