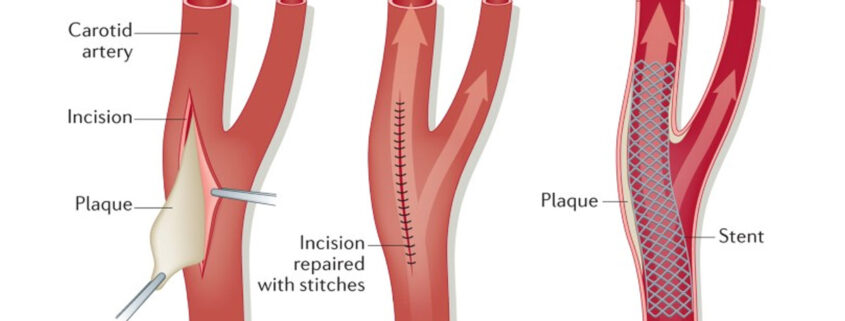
Complications of Carotid Endarterectomy (CEA) in the Postanesthesia Care Unit (PACU)
In patients with confirmed high-grade (70-99%) stenosis of the internal carotid artery, surgical carotid endarterectomy (CEA) is highly beneficial and has become the standard surgical treatment. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) found that patients who underwent surgery within 2 weeks after the onset of neurologic deficits had better neurologic outcomes, with a 17% absolute reduction in the risk of ipsilateral stroke at 2 years.
CEA is indicated for symptomatic 70-99% carotid stenosis and is three times as effective as medical therapy alone in reducing the incidence of stroke. In cases of moderate (50-69%) carotid stenosis, the benefit is less clear, and the patient’s risk factors for stroke must be weighed against the risk of perioperative complications in deciding between operative and medical therapy. Patients with lower-grade (< 50%) carotid stenosis do not benefit from surgical treatment.
Whereas the overall risk of perioperative stroke and death for patients undergoing CEA is 6.5%, the risk of a permanently disabling stroke and death is lower, at 2%. For the benefits of surgical therapy to outweigh those of medical therapy, the post-CEA complication rate must be less than 3%. In addition to perioperative stroke, serious complications that may develop after CEA include myocardial ischemia and infarction, hemodynamic instability, cranial nerve (CN) injuries, and bleeding resulting in neck hematomas and airway compromise.
Key considerations in the management of postoperative complications of CEA include the following:
- Surgical CEA is indicated in patients with severe (70-99%) carotid stenosis who are symptomatic with transient ischemic attacks (TIAs) or a non-disabling stroke and is three times as effective as medical therapy alone in reducing the incidence of stroke
- CEA in patients with symptomatic moderate (50-60%) carotid stenosis yields only a moderate reduction in the risk of stroke; the decision between operative and medical therapy in these patients must consider comorbidities and risk factors
- Hypertension is the most powerful yet treatable risk factor for postoperative stroke; careful control of blood pressure (BP) after CEA prevents cerebral hyperperfusion, weakening of the arteriotomy site, and impairment of cardiac function
- Ensuring hemostasis during wound closure reduces the risk of a postoperative wound hematoma
- In a time-sensitive critical situation when there is impending respiratory compromise or airway loss, opening the surgical incision to decompress the trachea and thereby facilitate airway management should always be considered.
- Urgent procedures and return to the operating room (OR) for a neurologic event or bleeding are associated with an increased risk of CN injury after CEA; however, given that the incidence of a permanent deficit is so low, the risk of CN injuries does not outweigh the benefit of stroke prevention after CEA
Postoperative Neurologic Complications
Most perioperative strokes occur during or within 12 hours after the surgical procedure. Ischemic strokes usually either are secondary to thrombosis or thromboembolism from the endarterectomy site or occur during intraoperative cross-clamping.
A hemorrhagic stroke after CEA is rare and is seen after the repair of critical stenosis in the presence of a distal infarct in a hypertensive patient. Other risk factors for intracerebral hemorrhage after CEA include the following:
- Advanced age
- Poor collateral flow
- Slow flow in the middle cerebral artery territory
Of the 1415 surgical patients enrolled in NASCET, only two suffered postoperative intracerebral hemorrhages.
Increased cerebral blood flow (CBF) to a previously under perfused territory can cause a phenomenon known as hyperperfusion syndrome, the symptoms of which include unilateral headache and altered mental status. Cerebral autoregulation is substantially impaired in the chronically hypoperfused territory; cerebral hyperperfusion from increased CBF, besides causing hemorrhagic strokes, can lead to brain edema, resulting in seizures.
The strict control of BP in the postoperative period can prevent or limit the severity of hyper perfusion syndrome and thus reduce the incidence of neurologic complications and death.
Management: Postoperative Neurologic Complications
At some institutions, admission of CEA patients to an intensive care unit (ICU) for close monitoring of hemodynamic status and neurologic function is standard procedure. However, it is unclear whether this resource-intensive practice significantly changes morbidity and mortality; the currently available data are insufficient to predict which patients will benefit from ICU admission.
O’Brien et al found that only a select few patients need ICU care after CEA; instead, they recommended observation in a recovery room, followed by admission to an intermediate care unit if the patient remains stable. To conserve hospital resources, only patients who have a prolonged recovery room course and are hemodynamically or neurologically unstable (states that would usually be evident in the first 2-3 hours after surgery) should be admitted to an ICU.
Evidence-based recommendations
Hypertension is the most prevalent and treatable risk factor for stroke, with isolated systolic hypertension further increasing the risk. Patients who undergo CEA should be monitored for at least 24 hours, regardless of whether they require ICU admission or not.
Even after the critical stenosis is corrected, blood vessels in the region distal to the stenosis remain maximally dilated. The chronic vasodilation results in loss of cerebral autoregulation, and perfusion becomes pressure-dependent; therefore, strict control of BP postoperatively is essential, and any elevation in BP must be aggressively treated, especially in patients who demonstrate symptoms of cerebral hyperperfusion.
If the patient remains hemodynamically and neurologically stable during the first 24 hours after surgery, discharge from the hospital is reasonable. Otherwise, patients should remain under observation until they are clinically stable.
In patients who show signs of an acute postoperative stroke, urgent surgical reexploration or cerebral angiography is recommended, with the goal of reopening occluded vessels, correcting the arterial repair, or both. The efficacy of this invasive approach in reversing stroke is unclear. In NASCET, 10 patients underwent emergency reoperation for a major hemispheric stroke; although occluded arteries were reopened in eight of them, none of the eight benefited.
On the other hand, a review examining 700 consecutive CEAs, in which 13 patients experienced major hemispheric defects, found that immediate surgical reexploration or cerebral angiography with reoperation based on the angiographic findings resulted in neurologic improvement in almost half of the 13. Despite neurologic improvement attributable to the reopening of the vessel, computed tomography (CT) still revealed new infarcts in almost all of them. Nevertheless, the authors concluded that urgent carotid repair may benefit a minority of selected patients who sustain a major stroke after CEA.
Postoperative Bleeding and Airway Compromise
Airway obstruction due to an enlarging neck hematoma after CEA is rare but potentially fatal. In the early postoperative period, patients complaining of unusual neck discomfort warrant special attention. Wound hematomas after CEA are relatively common, but fortunately, the majority are small and cause no problems. In NASCET, wound hematomas were documented in 5.5% of the patients and thus were a more common complication than a major stroke or death.
For large hematomas or those that continue to expand and result in airway loss or respiratory compromise, emergency treatment is indicated. If there is no airway compromise, the patient should return to the OR for emergency hematoma evacuation. However, if the airway is already obstructed by the hematoma, opening of the wound at the bedside is warranted.
Management: Postoperative Bleeding and Airway
Meticulous hemostasis during the closure of the wound after CEA is the most important factor in reducing the incidence of hematoma formation. Besides inadequate hemostasis, risk factors associated with hematoma formation include the following:
- Nonreversal of heparin
- Intraoperative hypotension
- Use of general anesthesia
- Perioperative statin use
- Use of a shunt
Hematoma formation, along with postoperative edema of the airway structures, makes airway management especially challenging in this emergency. Even in the absence of a hematoma formation, CT studies have shown that patients have increased airway edema, reducing the transverse airway diameter by as much as 75% in the postoperative period.
In a 10-year retrospective analysis from the Mayo Clinic College of Medicine that included 3245 patients who underwent CEA, Shakespeare et al found that the average interval between completion of the CEA and return to the OR for neck exploration was 6.0 ± 6.0 hours. Of the 3245 subjects, 44 (1.4%) required a return trip to the OR for neck exploration and hematoma evacuation; three of the 44 required surgical decompression to secure the airway, and one required an awake tracheostomy after a failed awake direct laryngoscopy.
In a time-sensitive critical situation where there is impending respiratory compromise or airway loss, the opening of the surgical incision to decompress the trachea and facilitate airway management should always be considered.
Evidence-based recommendations
After CEA, tracheal reintubation for airway protection in the setting of emergency hematoma evacuation may be challenging because of the distortion of the airway structures by the hematoma itself and by postoperative airway edema. In a large retrospective study from the Mayo Clinic College of Medicine, tracheal tube placement proved difficult in 40% of patients returning to the OR for hematoma evacuation, despite the absence of a history of difficult airway management for CEA earlier.
Although CEA is an increasingly routine procedure, recognition of the risk factors for the development of wound hematomas is nevertheless important for identifying patients who warrant closer postoperative monitoring in an ICU setting.
The laryngeal mask airway offers certain theoretical advantages, but the lack of data supporting its use, or the use of video laryngoscopy makes it difficult to compare these approaches with direct laryngoscopy and fiberoptic techniques. Each airway management technique has its own clinical and anatomic advantages and challenges. Accordingly, there is no standard approach.
If the patient is stable, it is reasonable to proceed with awake fiberoptic intubation to maintain spontaneous ventilation. However, if fiberoptic intubation is unsuccessful or the patient is unstable, the results from Shakespeare et al suggest that direct laryngoscopy with possible decompression of the trachea is likely to result in success.
Direct laryngoscopy may be done with the patient awake after topical anesthesia or after induction of general anesthesia. However, it is important to remember that induction of anesthesia coupled with the inability to intubate a patient with compromised physiologic reserve results in a life-threatening situation.
Regardless of the method used to secure the airway, the surgeon, the nursing team, and the anesthesiologist should all be prepared for the possibility that an emergency tracheostomy may prove necessary. In patients who are closely monitored and receiving timely airway assessment and management, this measure is rarely required.
Problem: Cranial Nerve Injuries
Although CN injuries are rarely considered in discussions of postoperative complications after a CEA, they are potentially serious when they do occur and can be life-threatening if they occur bilaterally. The reported incidence of CN injuries ranges from 2% to greater than 50%, with most such injuries being transient and secondary to neurapraxia caused by excessive retraction.
Because the clinical implications of a CN injury are like those of a minor stroke, proponents of carotid artery stenting claim that these injuries should be included in the composite endpoint of trials comparing stenting with CEA. However, the incidence of a permanent or disabling CN injury is very low, and for this reason, opponents of carotid artery stenting argue that this low injury risk should not detract from the significant benefit of stroke prevention conferred by CEA.
Management: Cranial Nerve Injuries
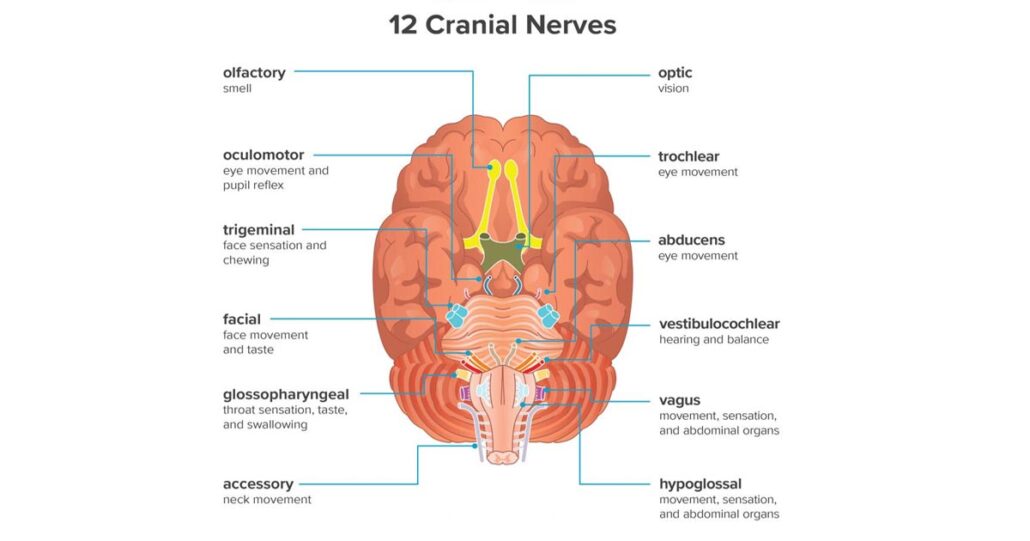
Injury to the facial nerve (CN VII), the glossopharyngeal nerve (CN IX), the vagus nerve (CN X), the spinal accessory nerve (CN XI), the hypoglossal nerve (CN XII), or the great auricular nerve is possible during CEA. A meta-analysis of 26 articles, corresponding to a total of 20,860 CEAs, identified urgent procedures and return to the OR for a neurologic event or bleeding as predictors of CN injury. Other independent predictors include the following:
- Age greater than 80 years
- Preoperative bleeding disorder
- Longer operating time, with an adjusted odds ratio of 1.15 for each 30-minute interval beyond an operating time of 90 minutes
The meta-analysis by Kakisis et al found no statistically significant association between CN injury and the type of anesthesia used.
Excessive retraction is the most common cause of CN injury; other, less common causes include the following:
- Blunt trauma
- Injury from forceps and arterial clamps
- Damage from electrocauterization
The vagus nerve appears to be the CN most injured during CEA, with a 3.99% incidence of injury and a 0.57% rate of permanent injury; the hypoglossal nerve is the next most injured CN.
It should be noted that the term permanent injury has variable meanings in this setting, given that different studies use different time frames. Some authors define a permanent deficit as one lasting longer than 12 months, whereas others consider a deficit permanent at 6 months. In addition, there have been reports of patients regaining function 3 years after a CN injury. Consequently, it is difficult at present to perform a true analysis of the incidence of permanent CN injury after CEA.
Data on the management of postoperative CN injuries are sparse. Prevention may be facilitated by thoroughly understanding the anatomy during surgical dissection to minimize traction and compression, as well as by using forceps, retractors, cautery, and arterial clamps with appropriate care. Perioperative dexamethasone also was shown to reduce the incidence of temporary CN injuries during CEAs; however, continued treatment in the presence of a CN injury had no effect on the incidence of permanent CN injuries.
Case Example 1
Clinical scenario
A 56-year-old woman with 90% stenosis of the right carotid artery has undergone an uncomplicated right CEA and has been admitted to the ICU for overnight monitoring, with the plan being to discharge her home the following day. In the ICU, the patient is hemodynamically stable. Approximately 4 hours later, it is noted that the right side of her neck is more edematous, with significant ecchymosis. She is taken back to the OR for neck exploration.
Because the patient is not in respiratory distress and shows no evidence of stridor, she is induced and placed under general anesthesia. However, direct laryngoscopy reveals significant tissue edema resulting in distorted anatomy, and the vocal cords cannot be visualized despite multiple attempts.
Resolution
Fortunately, mask ventilation proved feasible. The surgeon called for a surgical tracheostomy tray, which was immediately brought to the OR. A 6.0-mm endotracheal tube was inserted into the tracheostomy incision and connected to the ventilator. This was exchanged for a 6.0-mm Shiley cuffed tracheostomy tube. The patient’s oxygen saturation never decreased below 90%.
The surgeon opened the incision, evacuated the hematoma, and repaired the artery, leaving a drain in place before closing the incision again. The patient was taken back to the ICU and placed on the ventilator. She woke up the next morning, neurologically intact. She was seen by an otolaryngologist for management of her tracheostomy and was ultimately decannulated approximately 2 weeks later.
In this case, despite the absence of stridor or respiratory distress, the patient’s airway anatomy was found to be significantly distorted on direct laryngoscopy after induction of general anesthesia. It is difficult to predict the amount of bleeding and the degree of postoperative edema after CEA. Both events can significantly alter the airway anatomy. Therefore, anesthesiologists should proceed with caution and consider a more conservative approach (e.g., awake fiberoptic intubation), especially if the patient is stable but has progressive neck edema.
Opening the surgical incision to decompress the hematoma can improve the view of the airway, but a surgical tracheostomy tray with a capable surgeon should always be readily available as well.
Case Example 2
Clinical scenario
An obese 60-year-old man with insulin-dependent diabetes mellitus, hypertension, 99% stenosis of the right carotid artery, and 70% stenosis of the left carotid artery has undergone an uncomplicated right CEA and is being monitored in the post-anesthesia care unit (PACU), with the plan being to transfer him to a telemetry floor once he has fully recovered from general anesthesia and is hemodynamically stable.
On arrival in the PACU, the patient received labetalol5 mg IV for hypertension. During the second hour in the PACU, he displays left-side paralysis. Upon examination, the sensation is intact, but he lacks motor strength in the left arm and has only 3/5 strength (antigravity) in the left leg.
Resolution
A stroke code was called, and the patient was evaluated by the neurology team, who confirmed the physical examination findings. He was then taken from the PACU for an immediate head CT scan, the findings from which raised concern about an evolving stroke in the right middle cerebral artery territory. Neurointerventional radiology was consulted, and the patient was taken directly from the CT scanner to the interventional radiology suite for angiography. Cerebral angiography showed occlusion of the right middle cerebral artery in the proximal M1 territory.
An embolectomy was performed, reestablishing perfusion to the affected region. The patient was admitted to the ICU. Over the course of 24 hours, his neurologic examination results improve, and he regains full motor strength in the left leg.
Careful hemodynamic and neurologic monitoring after CEA, whether the patient is in the ICU or in the PACU, is vital for preventing and treating postoperative complications. Whether attempting to reverse a stroke is effective is unclear: Some studies show that a minority of patients benefit from such attempts, whereas the rest show that radiographic evidence of infarcts persists despite neurologic improvement. Nevertheless, signs and symptoms of acute stroke warrant immediate attention and intervention, with the goal of reopening the occluded vessel to prevent permanent major neurologic deficits.
Case Example 3
Clinical scenario
A 70-year-old woman with hypertension (treated with three different antihypertensive medications), a 30-pack-year smoking history, and a previous TIA resulting in the right hand and arm weakness are found to have 99% stenosis of the left internal carotid artery. After an uncomplicated left CEA, she is extubated and transferred to the ICU in stable condition.
The patient’s BP remains elevated, with systolic readings higher than 200 mm Hg. She is given multiple boluses of IV labetalol and hydralazine over the course of 4 hours to control her BP. Suddenly, however, she becomes increasingly somnolent and unresponsive with anisocoria. She is intubated for airway protection, and upon reexamination, her pupils are dilated and nonreactive to light. A portable head CT scan reveals a large temporoparietal intracerebral hemorrhage with 2-mm midline shift.
Resolution
The patient was hyperventilated and given IV mannitol 2 g/kg IV. Neurocritical care and neurosurgery were consulted. In view of the size of the hemorrhage with impending herniation, the consulting physicians advised the family that even with surgical intervention, the likelihood of meaningful recovery was very low. The family elected to transition the patient to comfort measures, and she died on a postoperative day 1.
This patient suffered a devastating hemorrhagic stroke after her CEA, which was precipitated by uncontrolled postoperative hypertension leading to cerebral hyperperfusion. Long-standing occlusive carotid disease results in compensatory dilation and loss of autoregulation in the distal cerebral vessels. CBF in abnormal, chronically under perfused areas is pressure-dependent, and in the postoperative state, it increases significantly. Uncontrolled hypertension after a CEA can result in rupture of the hyperperfused vessel, leading to hemorrhage.
In this patient, a more aggressive approach to BP control should have been taken. Instead of intermittent boluses of IV medications, a continuous infusion of an antihypertensive agent (e.g., nicardipine) should have been used.
What is surgical carotid endarterectomy (CEA)?
In patients with confirmed high-grade (70-99%) stenosis of the internal carotid artery, surgical carotid endarterectomy (CEA) is highly beneficial and has become the standard surgical treatment. The North American Symptomatic Carotid Endarterectomy Trial (NASCET) found that patients who underwent surgery within 2 weeks after the onset of neurologic deficits had better neurologic outcomes, with a 17% absolute reduction in the risk of ipsilateral stroke at 2 years.
What is the risk of complications from carotid endarterectomy (CEA)?
CEA is indicated for symptomatic 70-99% carotid stenosis and is three times as effective as medical therapy alone in reducing the incidence of stroke. In cases of moderate (50-69%) carotid stenosis, the benefit is less clear, and the patient’s risk factors for stroke must be weighed against the risk of perioperative complications in deciding between operative and medical therapy. Patients with lower-grade (< 50%) carotid stenosis do not benefit from surgical treatment.
Whereas the overall risk of perioperative stroke and death for patients undergoing CEA is 6.5%, the risk of a permanently disabling stroke and death is lower, at 2%. For the benefits of surgical therapy to outweigh those of medical therapy, the post-CEA complication rate must be less than 3%. In addition to perioperative stroke, serious complications that may develop after CEA include myocardial ischemia and infarction, hemodynamic instability, cranial nerve (CN) injuries, and bleeding resulting in neck hematomas and airway compromise.
What are the key considerations in the management of complications from carotid endarterectomy (CEA)?
Key considerations in the management of postoperative complications of CEA include the following:
- Surgical CEA is indicated in patients with severe (70-99%) carotid stenosis who are symptomatic with transient ischemic attacks (TIAs) or a non-disabling stroke and is three times as effective as medical therapy alone in reducing the incidence of stroke
- CEA in patients with symptomatic moderate (50-60%) carotid stenosis yields only a moderate reduction in the risk of stroke; the decision between operative and medical therapy in these patients must consider comorbidities and risk factors
- Hypertension is the most powerful yet treatable risk factor for postoperative stroke; careful control of blood pressure (BP) after CEA prevents cerebral hyperperfusion, weakening of the arteriotomy site, and impairment of cardiac function
- Ensuring hemostasis during wound closure reduces the risk of a postoperative wound hematoma
- In a time-sensitive critical situation when there is impending respiratory compromise or airway loss, opening the surgical incision to decompress the trachea and thereby facilitate airway management should always be considered.
- Urgent procedures and return to the operating room (OR) for a neurologic event or bleeding are associated with an increased risk of CN injury after CEA; however, given that the incidence of a permanent deficit is so low, the risk of CN injuries does not outweigh the benefit of stroke prevention after CEA
What are the neurologic complications of carotid endarterectomy (CEA)?
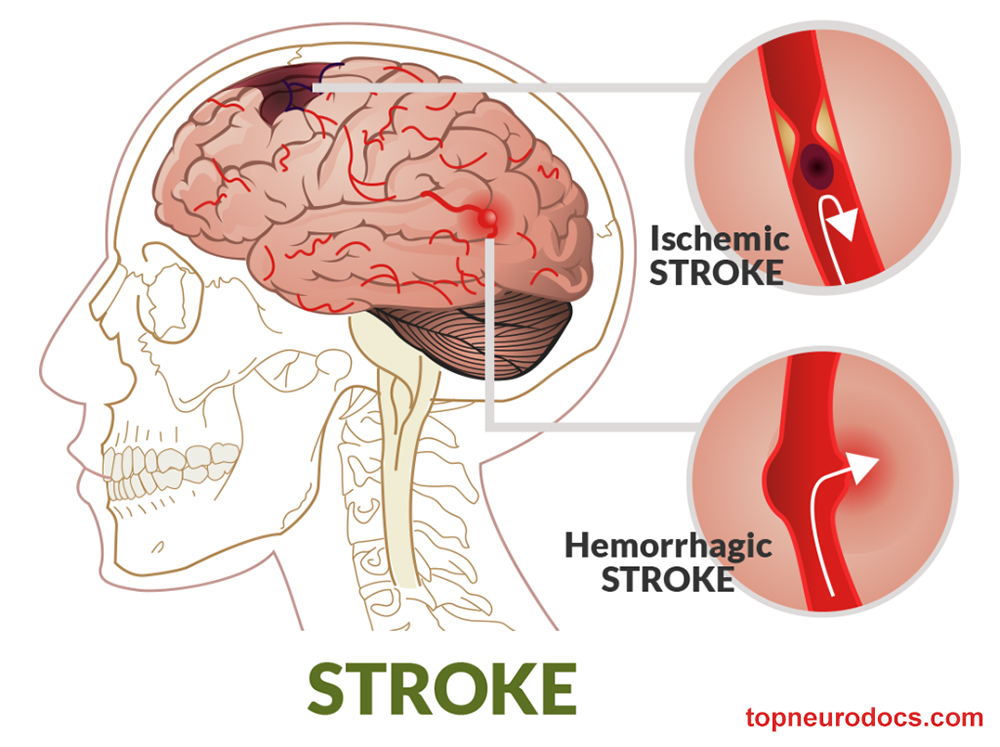
Most perioperative strokes occur during or within 12 hours after the surgical procedure. Ischemic strokes usually either are secondary to thrombosis or thromboembolism from the endarterectomy site or occur during intraoperative cross-clamping.
A hemorrhagic stroke after CEA is rare and is seen after the repair of critical stenosis in the presence of a distal infarct in a hypertensive patient. Other risk factors for intracerebral hemorrhage after CEA include the following:
- Advanced age
- Poor collateral flow
- Slow flow in the middle cerebral artery territory
Of the 1415 surgical patients enrolled in NASCET, only two suffered postoperative intracerebral hemorrhages.
Increased cerebral blood flow (CBF) to a previously under perfused territory can cause a phenomenon known as hyperperfusion syndrome, the symptoms of which include unilateral headache and altered mental status. Cerebral autoregulation is substantially impaired in the chronically hypoperfused territory; cerebral hyperperfusion from increased CBF, besides causing hemorrhagic strokes, can lead to brain edema, resulting in seizures.
The strict control of BP in the postoperative period can prevent or limit the severity of hyperperfusion syndrome and thus reduce the incidence of neurologic complications and death.
What is the role of the ICU in the management of neurologic complications of carotid endarterectomy (CEA)?
At some institutions, admission of CEA patients to an intensive care unit (ICU) for close monitoring of hemodynamic status and neurologic function is standard procedure. However, it is unclear whether this resource-intensive practice significantly changes morbidity and mortality; the currently available data are insufficient to predict which patients will benefit from ICU admission.
O’Brien et al found that only a select few patients need ICU care after CEA; instead, they recommended observation in a recovery room, followed by admission to an intermediate care unit if the patient remains stable. To conserve hospital resources, only patients who have a prolonged recovery room course and are hemodynamically or neurologically unstable (states that would usually be evident in the first 2-3 hours after surgery) should be admitted to an ICU.
How are the neurologic complications of carotid endarterectomy (CEA) treated?
Hypertension is the most prevalent and treatable risk factor for stroke, with isolated systolic hypertension further increasing the risk. Patients who undergo CEA should be monitored for at least 24 hours, regardless of whether they require ICU admission or not.
Even after the critical stenosis is corrected, blood vessels in the region distal to the stenosis remain maximally dilated. The chronic vasodilation results in loss of cerebral autoregulation, and perfusion becomes pressure-dependent; therefore, strict control of BP postoperatively is essential, and any elevation in BP must be aggressively treated, especially in patients who demonstrate symptoms of cerebral hyperperfusion.
If the patient remains hemodynamically and neurologically stable during the first 24 hours after surgery, discharge from the hospital is reasonable. Otherwise, patients should remain under observation until they are clinically stable.
In patients who show signs of an acute postoperative stroke, urgent surgical reexploration or cerebral angiography is recommended, with the goal of reopening occluded vessels, correcting the arterial repair, or both. The efficacy of this invasive approach in reversing stroke is unclear. In NASCET, 10 patients underwent emergency reoperation for a major hemispheric stroke; although occluded arteries were reopened in eight of them, none of the eight benefited.
On the other hand, a review examining 700 consecutive CEAs, in which 13 patients experienced major hemispheric defects, found that immediate surgical reexploration or cerebral angiography with reoperation based on the angiographic findings resulted in neurologic improvement in almost half of the 13. Despite neurologic improvement attributable to the reopening of the vessel, computed tomography (CT) still revealed new infarcts in almost all of them. Nevertheless, the authors concluded that urgent carotid repair may benefit a minority of selected patients who sustain a major stroke after CEA.
What are the possible postoperative bleeding and respiratory complications of carotid endarterectomy (CEA)?
Airway obstruction due to an enlarging neck hematoma after CEA is rare but potentially fatal. In the early postoperative period, patients complaining of unusual neck discomfort warrant special attention. Wound hematomas after CEA are relatively common, but fortunately, the majority are small and cause no problems. In NASCET, wound hematomas were documented in 5.5% of the patients and thus were a more common complication than a major stroke or death.
For large hematomas or those that continue to expand and result in airway loss or respiratory compromise, emergency treatment is indicated. If there is no airway compromise, the patient should return to the OR for emergency hematoma evacuation. However, if the airway is already obstructed by the hematoma, opening of the wound at the bedside is warranted.
What are the risk factors for hematoma formation following carotid endarterectomy (CEA)?
Meticulous hemostasis during the closure of the wound after CEA is the most important factor in reducing the incidence of hematoma formation. Besides inadequate hemostasis, risk factors associated with hematoma formation include the following:
- Nonreversal of heparin
- Intraoperative hypotension
- Use of general anesthesia
- Perioperative statin use
- Use of a shunt
Hematoma formation, along with postoperative edema of the airway structures, makes airway management especially challenging in this emergency. Even in the absence of a hematoma formation, CT studies have shown that patients have increased airway edema, reducing the transverse airway diameter by as much as 75% in the postoperative period.
In a 10-year retrospective analysis from the Mayo Clinic College of Medicine that included 3245 patients who underwent CEA, Shakespeare et al found that the average interval between completion of the CEA and return to the OR for neck exploration was 6.0 ± 6.0 hours. Of the 3245 subjects, 44 (1.4%) required a return trip to the OR for neck exploration and hematoma evacuation; three of the 44 required surgical decompression to secure the airway, and one required an awake tracheostomy after a failed awake direct laryngoscopy.
In a time-sensitive critical situation where there is impending respiratory compromise or airway loss, the opening of the surgical incision to decompress the trachea and facilitate airway management should always be considered.
How are postoperative bleeding and respiratory complications of carotid endarterectomy (CEA) treated?
After CEA, tracheal reintubation for airway protection in the setting of emergency hematoma evacuation may be challenging because of the distortion of the airway structures by the hematoma itself and by postoperative airway edema. In a large retrospective study from the Mayo Clinic College of Medicine, tracheal tube placement proved difficult in 40% of patients returning to the OR for hematoma evacuation, despite the absence of a history of difficult airway management for CEA earlier.
Although CEA is an increasingly routine procedure, recognition of the risk factors for the development of wound hematomas is nevertheless important for identifying patients who warrant closer postoperative monitoring in an ICU setting.
The laryngeal mask airway offers certain theoretical advantages, but the lack of data supporting its use, or the use of video laryngoscopy makes it difficult to compare these approaches with direct laryngoscopy and fiberoptic techniques. Each airway management technique has its own clinical and anatomic advantages and challenges. Accordingly, there is no standard approach.
If the patient is stable, it is reasonable to proceed with awake fiberoptic intubation to maintain spontaneous ventilation. However, if fiberoptic intubation is unsuccessful or the patient is unstable, the results from Shakespeare et al suggest that direct laryngoscopy with possible decompression of the trachea is likely to result in success.
Direct laryngoscopy may be done with the patient awake after topical anesthesia or after induction of general anesthesia. However, it is important to remember that induction of anesthesia coupled with the inability to intubate a patient with compromised physiologic reserve results in a life-threatening situation.
Regardless of the method used to secure the airway, the surgeon, the nursing team, and the anesthesiologist should all be prepared for the possibility that an emergency tracheostomy may prove necessary. In patients who are closely monitored and receiving timely airway assessment and management, this measure is rarely required.
What is the prevalence of cranial nerve injuries following carotid endarterectomy (CEA)?
Although CN injuries are rarely considered in discussions of postoperative complications after a CEA, they are potentially serious when they do occur and can be life-threatening if they occur bilaterally. The reported incidence of CN injuries ranges from 2% to greater than 50%, with most such injuries being transient and secondary to neurapraxia caused by excessive retraction.
Because the clinical implications of a CN injury are like those of a minor stroke, proponents of carotid artery stenting claim that these injuries should be included in the composite endpoint of trials comparing stenting with CEA. However, the incidence of a permanent or disabling CN injury is very low, and for this reason, opponents of carotid artery stenting argue that this low injury risk should not detract from the significant benefit of stroke prevention conferred by CEA.
What are the possible cranial nerve injuries caused by carotid endarterectomy (CEA)?
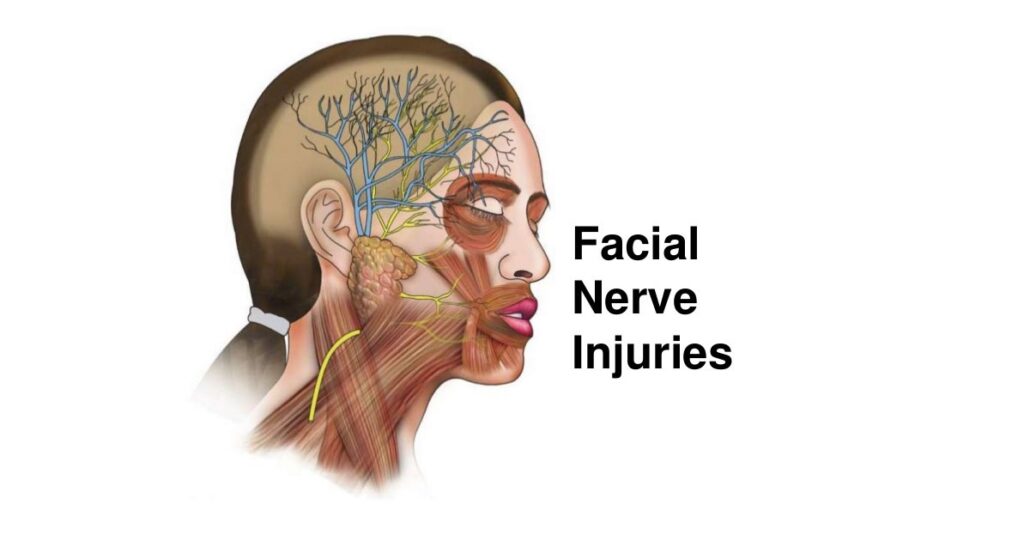
Injury to the facial nerve (CN VII), the glossopharyngeal nerve (CN IX), the vagus nerve (CN X), the spinal accessory nerve (CN XI), the hypoglossal nerve (CN XII), or the great auricular nerve is possible during CEA. A meta-analysis of 26 articles, corresponding to a total of 20,860 CEAs, identified urgent procedures and return to the OR for a neurologic event or bleeding as predictors of CN injury. Other independent predictors include the following:
- Age greater than 80 years
- Preoperative bleeding disorder
- Longer operating time, with an adjusted odds ratio of 1.15 for each 30-minute interval beyond an operating time of 90 minutes
The meta-analysis by Kakisis et al found no statistically significant association between CN injury and the type of anesthesia used.
How are cranial nerve injuries caused by carotid endarterectomy (CEA) treated?
Excessive retraction is the most common cause of CN injury; other, less common causes include the following:
- Blunt trauma
- Injury from forceps and arterial clamps
- Damage from electrocauterization
The vagus nerve appears to be the CN most injured during CEA, with a 3.99% incidence of injury and a 0.57% rate of permanent injury; the hypoglossal nerve is the next most injured CN.
It should be noted that the term permanent injury has variable meanings in this setting, given that different studies use different time frames. Some authors define a permanent deficit as one lasting longer than 12 months, whereas others consider a deficit permanent at 6 months. In addition, there have been reports of patients regaining function 3 years after a CN injury. Consequently, it is difficult at present to perform a true analysis of the incidence of permanent CN injury after CEA.
Data on the management of postoperative CN injuries are sparse. Prevention may be facilitated by thoroughly understanding the anatomy during surgical dissection to minimize traction and compression, as well as by using forceps, retractors, cautery, and arterial clamps with appropriate care. Perioperative dexamethasone also was shown to reduce the incidence of temporary CN injuries during CEAs; however, continued treatment in the presence of a CN injury had no effect on the incidence of permanent CN injuries.