Deep Vein Thrombosis and Pulmonary Embolism
Medicolegal Concerns
Pulmonary embolism (PE) is a common clinical problem that is associated with substantial morbidity and mortality. In the United States, more than 600,000 people have a pulmonary embolism each year, and more than 100,000 deaths could be prevented with proper diagnosis and treatment. The common pitfalls are as follows:
- Disregarding patient’s complaints of unexplained dyspnea as anxiety or hyperventilation
- Blaming complaints of unexplained chest pain on musculoskeletal pain
- Failing to recognize, diagnose, and treat deep vein thrombosis (DVT)
- Failing to initiate an appropriate diagnostic work-up in patients with symptoms consistent with pulmonary embolism
- Failing to initiate therapeutic anticoagulant therapy with heparin in patients suspected to have a pulmonary embolism, before imaging studies or other investigations
- Failure to advise of risk factors, such as smoking, pregnancy, and use of the oral contraceptive pill
- Failure to diagnose predisposing or associated conditions
Background
Pulmonary embolism is a common and potentially lethal condition. Most patients who succumb to pulmonary embolism do so within the first few hours of the event. Despite diagnostic advances, delays in pulmonary embolism diagnosis contribute to significant morbidity and mortality. As a cause of sudden death, massive pulmonary embolism is second only to sudden cardiac death.
The variability of presentation contributes to misdiagnosis of pulmonary embolism. The “classic” presentation with abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia is not often seen. Studies of patients who died unexpectedly of pulmonary embolism revealed that the patients had complained of nonspecific symptoms, often for weeks, before dying. Forty percent of these patients had been seen by a physician in the weeks prior to their death.
Patients with unexplained dyspnea, tachypnea, chest pain or the presence of risk factors for pulmonary embolism must undergo diagnostic tests until the diagnosis is confirmed or eliminated or an alternative diagnosis is found. Routine laboratory findings are often nonspecific and are not helpful in pulmonary embolism, although they may suggest another diagnosis. Pulmonary angiography had historically been the criterion standard for the diagnosis of pulmonary embolism, but with the improved sensitivity and specificity of CT angiography, it is now rarely performed.
In patients who survive a pulmonary embolism, recurrent embolism and death can be prevented with prompt diagnosis and treatment. If left untreated, approximately one third of patients who survive an initial pulmonary embolism die from a subsequent embolic episode.
Immediate full anticoagulation is mandatory for all patients suspected to have DVT or pulmonary embolism. Diagnostic investigations should not delay empirical anticoagulant therapy.
Long-term anticoagulation is critical to the prevention of recurrence of DVT or pulmonary embolism. A significant reduction in recurrence is associated with 3-6 months of anticoagulation.
Epidemiology
Pulmonary embolism is present in 60-80% of patients with DVT, even though more than half of these patients are asymptomatic. Pulmonary embolism is the third most common cause of death in hospitalized patients, with at least 650,000 cases occurring annually. Autopsy studies have shown that approximately 60% of patients who have died in the hospital had a pulmonary embolism, with the diagnosis having been missed in up to 70% of the cases. Prospective studies have demonstrated DVT in 10-13% of all medical patients placed on bed rest for 1 week, 29-33% of all patients in medical intensive care units, 20-26% of patients with pulmonary diseases who are placed on bed rest for 3 or more days, 27-33% of patients admitted to a critical care unit after a myocardial infarction, and 48% of patients who are asymptomatic after a coronary artery bypass graft.
Pulmonary embolism in elderly persons
Pulmonary embolism is increasingly prevalent among elderly patients, yet the diagnosis is missed more often in these patients than in younger ones because respiratory symptoms often are dismissed as being chronic. Even when the diagnosis is made, appropriate therapy has frequently been inappropriately withheld because of bleeding concerns. An appropriate diagnostic work-up and therapeutic anticoagulation with a careful risk-to-benefit assessment are recommended in this patient population.
Pulmonary embolism in pediatric patients
DVT and pulmonary embolism are rare in pediatric practice. In 1993, David et al identified 308 children reported in the medical literature from 1975-1993 with DVT of an extremity and/or pulmonary embolism. In 1986, Bernstein reported 78 episodes of pulmonary embolism per 100,000 hospitalized adolescents. Unselected autopsy studies in children estimate the incidence of pulmonary embolism from 0.05-3.7%.
However, among pediatric patients in whom DVT or pulmonary emboli do occur, these conditions are associated with significant morbidity and mortality. Various authors suggest that pulmonary embolism contributes to the death of affected children in approximately 30% of cases. (Others, however, have reported pulmonary embolism as a cause of death in less than 5% of affected children.)
Thromboembolic disease in pregnancy
A population-based study covering the years 1966-1995 collated the cases of DVT or pulmonary embolism in women during pregnancy or postpartum. The relative risk was 4.29, and the overall incidence of venous thromboembolism (absolute risk) was 199.7 incidents per 100,000 women-years. Among postpartum women, the annual incidence was 5 times higher than in pregnant women (511.2 vs 95.8 incidents per 100,000 women, respectively).
The incidence of DVT was 3 times higher than that of pulmonary embolism (151.8 vs 47.9 incidents, respectively, per 100,000 women). Pulmonary embolism was relatively less common during pregnancy than in the postpartum period (10.6 vs 159.7 incidents, respectively, per 100,000 women, respectively). A national review of severe obstetric complications from 1998-2005 found a significant increase in the rate of pulmonary embolism associated with the increasing rate of cesarean delivery.
Pulmonary embolism and postoperative mortality
Pulmonary embolism may account for 15% of all postoperative deaths. Leg amputations, and hip, pelvic, and spinal surgery are associated with the highest risk.
Etiology
Three primary influences predispose a patient to thrombus formation; these form the so-called Virchow triad, which consists of the following:
- Endothelial injury
- Stasis or turbulence of blood flow
- Blood hypercoagulability
Thrombosis usually originates as a platelet nidus on valves in the veins of the lower extremities. Further growth occurs by accretion of platelets and fibrin and progression to red fibrin thrombus, which may either break off and embolize or result in total occlusion of the vein. The endogenous thrombolytic system leads to partial dissolution; then, the thrombus becomes organized and is incorporated into the venous wall.
Pulmonary emboli usually arise from thrombi originating in the deep venous system of the lower extremities; however, they may rarely originate in the pelvic, renal, or upper extremity veins or the right heart chambers. After traveling to the lung, large thrombi can lodge at the bifurcation of the main pulmonary artery or the lobar branches and cause hemodynamic compromise. Smaller thrombi typically travel more distally, occluding smaller vessels in the lung periphery. These are more likely to produce pleuritic chest pain by initiating an inflammatory response adjacent to the parietal pleura. Most pulmonary emboli are multiple, and the lower lobes are involved more commonly than the upper lobes.
The causes for pulmonary embolism are multifactorial and are not readily apparent in many cases. The causes include the following:
Venous stasis
- Venous stasis leads to accumulation of platelets and thrombin in veins. Increased viscosity may occur due to polycythemia and dehydration, immobility, raised venous pressure in cardiac failure, or compression of a vein by a tumor.
Hypercoagulable states
- The complex and delicate balance between coagulation and anticoagulation is altered by many diseases, by obesity, or by trauma. It can also occur after surgery.
- Concomitant hypercoagulability may be present in disease states where prolonged venous stasis or injury to veins occurs.
- Hypercoagulable states may be acquired or congenital. Factor V Leiden mutation causing resistance to activated protein C is the most common risk factor. Factor V Leiden mutation is present in up to 5% of the normal population and is the most common cause of familial thromboembolism.
- Primary or acquired deficiencies in protein C, protein S, and antithrombin III are other risk factors. The deficiency of these natural anticoagulants is responsible for 10% of venous thrombosis in younger people.
Immobilization
- Immobilization leads to local venous stasis by accumulation of clotting factors and fibrin, resulting in thrombus formation. The risk of pulmonary embolism increases with prolonged bed rest or immobilization of a limb in a cast.
- In the Prospective Investigation of Pulmonary Embolism Diagnosis II (PIOPED II) study, immobilization (usually because of surgery) was the risk factor most commonly found in patients with pulmonary embolism.
Surgery and trauma
A prospective study by Geerts and colleagues indicated that major trauma was associated with a 58% incidence of DVT in the lower extremities and an 18% incidence in proximal veins.
Surgical and accidental traumas predispose patients to venous thromboembolism by activating clotting factors and causing immobility. Pulmonary embolism may account for 15% of all postoperative deaths. Leg amputations, and hip, pelvic, and spinal surgery are associated with the highest risk.
Fractures of the femur and tibia are associated with the highest risk of fracture-related pulmonary embolism, followed by pelvic, spinal, and other fractures. Severe burns also carry a high risk of DVT or pulmonary embolism.
Pregnancy
The incidence of thromboembolic disease in pregnancy has been reported to range from 1 case in 200 deliveries to 1 case in 1400 deliveries. Fatal events are rare, with 1-2 cases occurring per 100,000 pregnancies.
Oral contraceptives and estrogen replacement
Estrogen-containing birth control pills have increased the occurrence of venous thromboembolism in healthy women. The risk is proportional to the estrogen content and is increased in postmenopausal women on hormonal replacement therapy. The relative risk is 3-fold, but the absolute risk is 20-30 cases per 100,000 persons per year.
Malignancy
Malignancy has been identified in 17% of patients with venous thromboembolism. Pulmonary emboli have been reported to occur in association with solid tumors, leukemias, and lymphomas. This is probably independent of the indwelling catheters often used in such patients. The neoplasms most commonly associated with pulmonary embolism, in descending order of frequency, are pancreatic carcinoma; bronchogenic carcinoma; and carcinomas of the genitourinary tract, colon, stomach, and breast.
Hereditary factors
Hereditary factors associated with the development of pulmonary embolism include the following:
- Antithrombin III deficiency
- Protein C deficiency
- Protein S deficiency
- Factor V Leiden (most common genetic risk factor for thrombophilia)
- Plasminogen abnormality
- Plasminogen activator abnormality
- Fibrinogen abnormality
- Resistance to activated protein C
Acute medical illness
Acute medical illnesses associated with the development of pulmonary embolism include the following:
- AIDS (lupus anticoagulant)
- Bechet disease
- Congestive heart failure (CHF)
- Myocardial infarction
- Polycythemia
- Systemic lupus erythematosus
- Ulcerative colitis
HIV Infection
Individuals with HIV infection are more likely to have clinically detected thromboembolic disease. The risk of developing a pulmonary embolism or DVT is increased 40% in these individuals.
Sickle cell disease
Sickle cell disease often creates a diagnostic difficulty in pulmonary embolism. A chest infection is often the presenting symptom. Hypoxemia, dehydration, and fever lead to intravascular sludging within pulmonary (among others) vasculature. This promotes a vicious cycle, further exacerbating local hypoxemia, ultimately leading to local tissue infarction. This process is further worsened by bone marrow infarction, which may cause the release of fat emboli that lodge in the pulmonary circulation.
Pulmonary embolism in children
In contrast to adults, most children (98%) diagnosed with pulmonary emboli have an identifiable risk factor or a serious underlying disorder.
In 1993, David et al reported that 21% of children with DVT and/or pulmonary emboli had an indwelling central venous catheter. Additional series have reported the presence of central lines in as many as 36% of patients. A clot may form as a fibrin sleeve that encases the catheter. When the catheter is removed, the fibrin sleeve is often dislodged, releasing a nidus for embolus formation. In another scenario, a thrombus may adhere to the vessel wall adjacent to the catheter.
David and colleagues also reported that 5-10% of children with venous thromboembolic disease have inherited disorders of coagulation, such as antithrombin III, protein C, or protein S deficiency. In 1997, Nuss et al reported that 70% of children with a diagnosis of pulmonary embolism have antiphospholipid antibodies or coagulation-regulatory protein abnormalities. However, this was a small study in a population with clinically recognized pulmonary emboli; hence, its applicability to the broader pediatric population is uncertain.
A study reported that major thrombosis or pulmonary embolism was present in more than 33% of children treated with long-term hyperalimentation and that pulmonary embolism was the major cause of death in 30% of these children. Fat embolization may exacerbate this clinical picture.
Dehydration, especially hyperosmolar dehydration, is typically observed in younger infants with pulmonary emboli.
Classification
When a pulmonary embolism is identified, it is characterized as acute or chronic. In terms of pathologic diagnosis, an embolus is acute if it is situated centrally within the vascular lumen or if it occludes a vessel (vessel cutoff sign). Acute pulmonary embolism commonly causes distention of the involved vessel. An embolus is chronic if it is eccentric and contiguous with the vessel wall, it reduces the arterial diameter by more than 50%, evidence of recanalization within the thrombus is present, and an arterial web is present.
A pulmonary embolism is also characterized as central or peripheral, depending on the location or the arterial branch involved. Central vascular zones include the main pulmonary artery, the left and right main pulmonary arteries, the anterior trunk, the right and left interlobar arteries, the left upper lobe trunk, the right middle lobe artery, and the right and left lower lobe arteries.
A pulmonary embolus is characterized as massive when it involves both pulmonary arteries, and when it results in hemodynamic compromise. Peripheral vascular zones include the segmental and subsegmental arteries of the right upper lobe, the right middle lobe, the right lower lobe, the left upper lobe, the lingual, and the left lower lobe.
Knowledge of bronchovascular anatomy is the key to the accurate interpretation of CT scans obtained for the evaluation of pulmonary embolism. A systematic approach to identifying all vessels is important. The bronchovascular anatomy has been described upon the basis of the segmental anatomy of the lungs. The segmental arteries are seen near the accompanying branches of the bronchial tree and are situated either medially (in the upper lobes) or laterally (in the lower lobes, lingual, and right middle lobe).
Pathophysiology
Pulmonary embolism is not a disease; rather, pulmonary embolism is a complication of venous thromboembolism, most commonly deep vein thrombosis.
Pulmonary emboli usually arise from thrombi that originate in the deep venous system of the lower extremities; however, they rarely also originate in the pelvic, renal, upper extremity veins, or the right heart chambers. After traveling to the lung, large thrombi can lodge at the bifurcation of the main pulmonary artery or the lobar branches and cause respiratory and hemodynamic compromise.
Respiratory consequences
Acute respiratory consequences of pulmonary embolism include the following:
- Increased alveolar dead space
- Hypoxemia
- Hyperventilation
Additional consequences that may occur include regional loss of surfactant and pulmonary infarction. Arterial hypoxemia is a frequent, but not universal, finding in patients with acute embolism. The mechanisms of hypoxemia include ventilation-perfusion mismatch, intrapulmonary shunts, reduced cardiac output, and intracardiac shunt via a patent foramen ovale. Pulmonary infarction is an uncommon consequence because of the bronchial arterial collateral circulation.
Hemodynamic consequences
Pulmonary embolism reduces the cross-sectional area of the pulmonary vascular bed, resulting in increased pulmonary vascular resistance, which, in turn, increases the right ventricular afterload. If the afterload is increased severely, right ventricular failure may ensue. In addition, the humoral and reflex mechanisms contribute to the pulmonary arterial constriction. Following the initiation of anticoagulant therapy, the resolution of emboli usually occurs rapidly during the first 2 weeks of therapy; however, it can persist on chest imaging studies for months to years. Chronic pulmonary hypertension may occur with the failure of the initial embolus to undergo lyses or in the setting of recurrent thromboemboli.
History
The classic presentation of pulmonary embolism is the abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia. However, most patients with pulmonary embolism have no obvious symptoms at presentation. Rather, symptoms may vary from sudden catastrophic hemodynamic collapse to gradually progressive dyspnea. The diagnosis of pulmonary embolism should be suspected in patients with respiratory symptoms unexplained by an alternative diagnosis.
Studies of patients who died unexpectedly from PE have revealed that often these individuals complained of nagging symptoms for weeks before death. Forty percent of these patients had been seen by a physician in the weeks prior to their death.
The following risk factors can be indications for the presence of pulmonary embolism:
- Venous stasis
- Hypercoagulable states
- Immobilization
- Surgery and trauma
- Pregnancy
- Oral contraceptives and estrogen replacement
- Malignancy
- Hereditary factors resulting in a hypercoagulable state
- Acute medical illness
- Drug abuse (intravenous [IV] drugs)
- Drug-induced lupus anticoagulant
- Hemolytic anemias
- Heparin-associated thrombocytopenia
- Homocystinemia
- Homocystinuria
- Hyperlipidemias
- Phenothiazines
- Thrombocytosis
- Varicose veins
- Venography
- Venous pacemakers
- Warfarin (first few days of therapy)
- Inflammatory bowel disease
- Sleep-disordered breathing
The PIOPED II study listed the following indicators for pulmonary embolism:
- Travel of 4 hours or more in the past month
- Immobilization
- Surgery within the last 3 months
- Malignancy, especially lung cancer
- Current or history of thrombophlebitis
- Trauma to the lower extremities and pelvis during the past 3 months
- Smoking
- Central venous instrumentation within the past 3 months
- Stroke, paresis, or paralysis
- Prior pulmonary embolism
- Heart failure
- Chronic obstructive pulmonary disease
Physical Examination
Physical examination findings are quite variable in pulmonary embolism and, for convenience, may be grouped into four categories as follows:
- Massive pulmonary embolism
- Acute pulmonary infarction
- Acute embolism without infarction
- Multiple pulmonary emboli or thrombi
The presentation of pulmonary embolism may vary from sudden catastrophic hemodynamic collapse to gradually progressive dyspnea. (Prior poor cardiopulmonary status of the patient is an important factor leading to hemodynamic collapse.) Most patients with pulmonary embolism have no obvious symptoms at presentation. In contrast, patients with symptomatic DVT commonly have pulmonary embolism confirmed in diagnostic studies in the absence of pulmonary symptoms.
Patients with pulmonary embolism may present with atypical symptoms. In such cases, a strong suspicion of pulmonary embolism based on the presence of risk factors can lead to consideration of pulmonary embolism in the differential diagnosis. These symptoms include the following:
- Seizures
- Syncope
- Abdominal pain
- Fever
- Productive cough
- Wheezing
- Decreasing level of consciousness
- New onset of atrial fibrillation
- Flank pain
- Delirium (in elderly patients)
The diagnosis of pulmonary embolism should be sought actively in patients with respiratory symptoms unexplained by an alternative diagnosis. The symptoms of pulmonary embolism are nonspecific; therefore, a high index of suspicion is required, particularly when a patient has risk factors for the condition.
In patients with recognized pulmonary embolism, the incidence of physical signs has been reported as follows:
- Tachypnea (respiratory rate >16/min) – 96%
- Rales – 58%
- Accentuated second heart sound – 53%
- Tachycardia (heart rate >100/min) – 44%
- Fever (temperature >37.8°C [100.04ºF]) – 43%
- Diaphoresis – 36%
- S3 or S4 gallop – 34%
- Clinical signs and symptoms suggesting thrombophlebitis – 32%
- Lower extremity edema – 24%
- Cardiac murmur – 23%
- Cyanosis – 19%
The PIOPED study reported the following incidence of common symptoms of pulmonary embolism:
- Dyspnea (73%)
- Pleuritic chest pain (66%)
- Cough (37%)
- Hemoptysis (13%)
Fever(temperature >37.8°C [100.04ºF]) is found in 43% of patients; however, temperatures higher than 39.5°C (103.1º) F not from pulmonary embolism. Chest wall tenderness upon palpation, without a history of trauma, may be the sole physical finding in rare cases.
Pleuritic chest pain without other symptoms or risk factors may be a presentation of pulmonary embolism. Pleuritic chest pain is reported to occur in as many as 84% of patients with pulmonary emboli. Its presence suggests that the embolus is located more peripherally and thus may be smaller.
Pulmonary embolism has been diagnosed in 21% of young, active patients who come to emergency departments (EDs) complaining only of pleuritic chest pain. These patients usually lack any other classical signs, symptoms, or known risk factors for pulmonary thromboembolism. Such patients often are dismissed inappropriately with an inadequate work-up and a nonspecific diagnosis, such as musculoskeletal chest pain or pleurisy.
Massive pulmonary embolism
Patients with massive pulmonary embolism are in shock. They have systemic hypotension, poor perfusion of the extremities, tachycardia, and tachypnea. In addition, patients appear weak, pale, sweaty, and oliguric and develop impaired mentation.
Signs of pulmonary hypertension, such as palpable impulse over the second left intercostal space, loud P2, right ventricular S3 gallop, and a systolic murmur louder on inspiration at the left sternal border (tricuspid regurgitation), may be present.
Massive pulmonary embolism has been defined by hemodynamic parameters and evidence of myocardial injury rather than anatomic findings because the former is associated with adverse outcomes. Although previous studies of CT scans in the diagnosis of pulmonary embolus suggested that central obstruction was not associated with adverse outcomes, a new multicenter study clarifies this observation. Vedovati et al. found no association between central obstruction and death or clinical deterioration in 579 patients with pulmonary embolus. However, when a subset of 516 patients who were hemodynamically stable was assessed, central localization of emboli was found to be an independent mortality risk factor while distal localization was inversely associated with adverse events. Thus, anatomic findings by CT scan may be important in assessing risk in hemodynamically stable patients with pulmonary embolus.
Acute pulmonary infarction
Approximately 10% of patients have peripheral occlusion of a pulmonary artery, causing parenchymal infarction. These patients present with acute onset of pleuritic chest pain, breathlessness, and hemoptysis. Although the chest pain may be clinically indistinguishable from ischemic myocardial pain, normal ECG findings and no response to nitroglycerin rules out myocardial pain. Patients with acute pulmonary infarction have decreased excursion of the involved hemithorax, palpable or audible pleural friction rub, and even localized tenderness. Signs of pleural effusion, such as dullness to percussion and diminished breath sounds, may be present.
Acute embolism without infarction
Patients with acute embolism without infarction have nonspecific physical signs that may easily be secondary to another disease process. Tachypnea and tachycardia frequently are detected, pleuritic pain sometimes may be present, crackles may be heard in the area of embolization, and local wheeze may be heard rarely.
Multiple pulmonary emboli or thrombi
Patients with pulmonary emboli and thrombi have physical signs of pulmonary hypertension and Cor pulmonale. Patients may have elevated jugular venous pressure, right ventricular heave, palpable impulse in the left second intercostal space, right ventricular S3 gallop, systolic murmur over the left Sternal border that is louder during inspiration, hepatomegaly, ascites, and dependent pitting edema. These findings are not specific for pulmonary embolism and require a high index of suspicion for pursuing appropriate diagnostic studies.
Pulmonary emboli in children
Many physical findings are typically less marked in children than they are in adults, presumably because children have greater hemodynamic reserve and, thus, are better able to tolerate the significant hemodynamic and pulmonary changes.
Because of the rarity of pulmonary emboli in children, these patients are probably underdiagnosed. For the same reason, much of the information pertaining to the diagnosis and management of pulmonary embolism has been derived from adult practice.
Cough is present in approximately 50% of children with pulmonary emboli; tachypnea occurs with the same frequency. Hemoptysis is a feature in a minority of children with pulmonary emboli, occurring in about 30% of cases. Crackles are heard in a minority of cases.
Cyanosis and hypoxemia are not prominent features of pulmonary embolism. If present, cyanosis suggests a massive embolism leading to a marked ventilation-perfusion (V/Q) mismatch and systemic hypoxemia. Some case reports have described massive pediatric pulmonary embolism with normal saturation.
A pleural rub is often associated with pleuritic chest pain and indicates an embolism in a peripheral location in the pulmonary vasculature. Signs that indicate pulmonary hypertension and right ventricular failure include a loud pulmonary component of the second heart sound, right ventricular lift, distended neck veins, and hypotension. An increase in pulmonary arterial pressure is reportedly not evident until at least 60% of the vascular bed has been occluded.
A gallop rhythm signifies ventricular failure, while peripheral edema is a sign of congestive heart failure. Various heart murmurs may be audible, including a tricuspid regurgitant murmur signifying pulmonary hypertension.
Fever is an unusual sign that is nonspecific, and diaphoresis is a manifestation of sympathetic arousal. Signs of other organ involvement in patients with sickle cell disease would be elicited, such as sequestration crisis, priapism, anemia, and stroke.
Diagnostic Considerations
The “classic” presentation of pulmonary embolism, with abrupt onset of pleuritic chest pain, shortness of breath, and hypoxia, is rarely seen.
The variability of presentation for pulmonary embolism (PE) sets the patient and clinician up for potentially missing the diagnosis. Such missed diagnoses occur in approximately 400,000 patients in the United States per year; approximately 100,000 deaths could be prevented with proper diagnosis and treatment. Studies of patients who died unexpectedly from PE have revealed that the patients complained of nagging symptoms, often for weeks, before dying. Forty percent of these patients had been seen by a physician in the weeks prior to their death.
Patients suspected of having pulmonary embolism—because of unexplained dyspnea, tachypnea, or chest pain or the presence of risk factors for pulmonary embolism—must undergo diagnostic tests until the diagnosis is ascertained or eliminated or an alternative diagnosis is confirmed. Further, routine laboratory findings are nonspecific and are not helpful in pulmonary embolism, although they may suggest another diagnosis.
A hypercoagulation work-up should be performed if no obvious cause for embolic disease is apparent. This may include screening for conditions such as the following:
- Antithrombin III deficiency
- Protein C or protein S deficiency
- Lupus anticoagulant
- Homocystinuria
- Occult neoplasm
- Connective tissue disorders
The differential diagnoses are extensive, and they should be considered carefully with any patient thought to have pulmonary embolism. These patients also should have an alternative diagnosis confirmed, or pulmonary embolism should be excluded, before discontinuing the work-up. Additional problems to be considered include the following:
Differential Diagnoses
- Acute Coronary Syndrome
- Acute Pericarditis
- Acute Respiratory Distress Syndrome
- Angina Pectoris
- Anxiety Disorders
- Aortic Stenosis
- Atrial Fibrillation
- Cardiogenic Shock
- Cor Pulmonale
- Dilated Cardiomyopathy
- Emphysema
- Fat Embolism
- Hypersensitivity Pneumonitis
- Hyperventilation
- Idiopathic Pulmonary Arterial Hypertension
- Lung trauma
- Mediastinitis, acute
- Mitral Stenosis
- Musculoskeletal pain
- Myocardial Infarction
- Pleuritis
- Pneumothorax Imaging
- Pulmonary Arterial Hypertension
- Pulmonary Arteriovenous Fistulae
- Restrictive Cardiomyopathy
- Salicylate intoxication
- Silicone pulmonary embolism
- Sudden Cardiac Death
- Superior Vena Cava Syndrome in Emergency Medicine
- Syncope
Clinical Scoring Systems
Evidence-based literature supports the practice of determining the clinical probability of pulmonary embolism before proceeding with testing. One study assessed the performance of four clinical decision rules in addition to D-dimer testing to exclude acute PE. All four rules, Wells rule, simplified Wells rule, revised Geneva score and simplified revised Geneva score, showed similar performance for excluding acute PE when combined with a normal D-dimer result.
D-Dimer Follow-Up on Low-to-Moderate Pretest Probability
When clinical prediction rule results indicate that the patient has a low or moderate pretest probability of pulmonary embolism, D-dimer testing may be the next step.
D-Dimer, a degradation product produced by plasmin-mediated proteases of cross-linked fibrin, is measured by a variety of assay types, including quantitative, semiquantitative, and qualitative rapid enzyme-linked immunosorbent assays (ELISAs); quantitative and semiquantitative latex; and whole-blood assays. A systematic review of prospective studies of high methodological quality concluded that the ELISAs—especially the quantitative rapid ELISA—dominate the comparative ranking among the D-dimer assays for sensitivity and negative likelihood ratio.
Negative results on a high-sensitivity D-dimer test in a patient with a low pretest probability of pulmonary embolism indicate a low likelihood of venous thromboembolism and reliably exclude pulmonary embolism. A large, prospective, randomized trial found that in patients with a low probability of pulmonary embolism who had negative D-dimer results, foregoing additional diagnostic testing was not associated with an increased frequency of symptomatic venous thromboembolism during the subsequent 6 months.
In a 2012 prospective cohort study, a Wells score of 4 or less combined with a negative qualitative D-dimer test was shown to safely exclude pulmonary embolism in primary care patients.
D-dimer testing is most reliable for excluding pulmonary embolism in younger patients who have no associated comorbidity or history of venous thromboembolism and whose symptoms are of short duration. However, it is of questionable value in patients who are older than 80 years, who are hospitalized, who have cancer, or who are pregnant, because nonspecific elevation of D-dimer concentrations is common in such patients.
D-dimer testing should not be used when the clinical probability of pulmonary embolism is high, because the test has low negative predictive value in such cases.
Combining D-dimer results with measurement of the exhaled end-tidal ratio of carbon dioxide to oxygen (etCO2/O2) can be useful for diagnosis of pulmonary embolism. Kline et al found that, in moderate-risk patients with a positive D-dimer (>499 ng/ml), an etCO2/O2< 0.28 significantly increased the probability of finding segmental or larger pulmonary embolism on computed tomography multidetector-row pulmonary angiography, while an etCO2/O2) >0.45 predicted the absence of segmental or larger pulmonary embolism.
Because of the poor specificity, positive D-dimer measurements are not helpful in confirming the diagnosis of venous thromboembolic disease. However, a positive D-dimer measurement may lead to consideration of venous thromboembolic disease in the differential diagnosis in selected patients. In addition, the use of D-dimers in children is not well studied. A small pediatric series reported that D-dimer measurements are negative in 40% of patients. A retrospective series reported an elevated D-dimer in 86% of patients at presentation.
Ischemia-Modified Albumin levels
A potential alternative to D-dimer testing is an assessment of the ischemia-modified albumin (IMA) level, which data suggest is 93% sensitive and 75% specific for pulmonary embolism. Notably, in a study comparing the prognostic value of IMA to D-dimer testing, IMA assessment in combination with Wells and Geneva probability scores appeared to positively impact overall sensitivity and negative predictive value. The positive predictive value of IMAis better than D-dimer. However, it should not be used alone.
White Blood Cell Count
The white blood cell (WBC) count may be normal or elevated in patients with pulmonary embolism, with a WBC count as high as 20,000 being not uncommon in patients with this condition.
Arterial Blood Gases
Arterial blood gas determinations characteristically reveal hypoxemia, hypercapnia, and respiratory alkalosis; however, the predictive value of hypoxemia is quite low. The PaO2 and the calculation of alveolar-arterial oxygen gradient contribute to the diagnosis in a general population thought to have a pulmonary embolism. Nonetheless, in high-risk settings such as patients in postoperative states in whom other respiratory conditions can be ruled out, a low PaO2 in conjunction with dyspnea may have a strong positive predictive value.
The PO2 on arterial blood gas analysis (ABG) has a zero or even negative predictive value in a typical population of patients in whom pulmonary embolism is suspected clinically. This is contrary to what has been taught in many textbooks, and even though it seems counterintuitive, it is demonstrably true. This is because other etiologies that masquerade as pulmonary embolism are more likely to lower the PO2 than pulmonary embolism. In fact, because other diseases that may masquerade as pulmonary embolism (e.g., chronic obstructive pulmonary disease [COPD], pneumonia, CHF) affect oxygen exchange more than does pulmonary embolism, the blood oxygen level often has an inverse predictive value for pulmonary embolism.
In most settings, less than half of all patients with symptoms suggestive of pulmonary embolism have a pulmonary embolism. In such a population, if any reasonable level of PaO2 is chosen as a dividing line, the incidence of pulmonary embolism will be higher in the group with a PaO2 above the dividing line than in the group whose PaO2 is below the divider. This is a specific example of a general truth that may be demonstrated mathematically for any test finding with a Gaussian distribution and a population incidence of less than 50%.
Conversely, in a patient population with a very high incidence of pulmonary embolism and a lower incidence of other respiratory ailments (e.g., postoperative orthopedic patients with sudden onset of shortness of breath), a low PO2 has a strongly positive predictive value for pulmonary embolism.
The discussion above holds true not only for arterial PO2, but also for the alveolar-arterial oxygen gradient and for the oxygen saturation level as measured by pulse oximetry. Pulse oximetry is extremely insensitive, is normal in the majority of patients with pulmonary embolism and should not be used to direct a diagnostic work-up.
Troponin levels
Serum troponin levels can be elevated in up to 50% of patients with a moderate to large pulmonary embolism, presumptively due to acute right ventricular myocardial stretch.
Although troponin assessment is not currently recommended as part of the diagnostic work-up, studies have shown that elevated troponin levels in the setting of pulmonary embolism correlate with increased mortality. However, further studies need to be performed to identify subsets of patients with pulmonary embolism who might benefit from this testing.
A meta-analysis of Jimenez et al suggested that in acute symptomatic pulmonary embolism, elevated troponin levels do not distinguish between patients who are at high risk for death and those who are at low risk. Pooled results from studies including 1366 normotensive patients with acute symptomatic pulmonary embolism showed that elevated troponin levels were associated with 4.26 increased odds of overall mortality. Summary receiver operating characteristic curve analysis showed a relationship between the sensitivity and specificity of troponin levels to predict overall mortality.
Serum troponin, although seemingly marginal for purposes of diagnosis of pulmonary embolism, may contribute significantly to the ability to stratify patients by risk for short-term death or adverse outcome events when they reach the ED. In patients with pulmonary embolism and normal blood pressure specifically, elevated serum troponin level has been associated with right ventricular overload.
Leptin is another cardiovascular risk factor that may be associated with outcome in acute pulmonary embolism. Dellas et al conducted a prospective analysis of 264 patients with acute pulmonary embolus and found that serum leptin levels were inversely associated with the risk of adverse outcomes. Further study will be needed to confirm these findings and determine the clinical utility of leptin measurement.
Brain Natriuretic Peptide
Although the brain natriuretic peptide (BNP) tests are neither sensitive nor specific, patients with pulmonary embolism tend to have higher BNP levels. BNP testing had a sensitivity and specificity of only 60% and 62%, respectively, in a case-control study of 2213 hemodynamically stable patients with suspected acute pulmonary embolism.
Elevated levels of BNP or of its precursor, N -terminal pro-brain natriuretic peptide (NT-proBNP), do correlate with an increased risk of subsequent complications and mortality in patients with acute pulmonary embolism. One meta-analysis revealed that patients with a BNP level greater than 100 mpg/ml or an NT-proBNP level greater than 600 ng/l had an all-cause in-hospital mortality rate 6- and 16-fold higher than those below these cutoffs, respectively. In a second smaller observational study, serum BNP levels greater than 90 pg. /ml were associated with a higher rate of complications, such as the need for cardiopulmonary resuscitation, need for mechanical ventilation, need for vasopressor therapy, and death.
BNP testing is not currently recommended as part of the standard evaluation of acute pulmonary embolism, and future studies may aid in defining its role in this setting.
Elevated levels of brain-type Natriuretic peptides (BNP) may also provide prognostic information. A meta-analysis demonstrated a significant association between elevated N-terminal–pro-BNP (NT-pro-BNP) and right ventricular function in patients with pulmonary embolism (P< .001), leading to an increased risk for the complicated in-hospital course. Importantly, increased NT-pro-BNP alone does not justify more invasive treatment.
A recent study by Scherz et al analyzed a large sample of patients hospitalized with acute pulmonary embolism. Hyponatremia at presentation was common and was associated with a higher risk of 30-day mortality and readmission.
Venography
Venography is the criterion standard for diagnosing DVT. With the advent of noninvasive imaging, it has become less common in pediatric and adult practice.
Angiography
Pulmonary angiography is the historical criterion standard for the diagnosis of pulmonary embolism. Following the injection of iodinated contrast, anteroposterior, lateral, and oblique studies are performed on each lung. Positive results consist of a filling defect or sharp cutoff of the affected artery. Nonocclusive emboli are described as having a tram-track appearance. Abnormal findings on V/Q scans performed prior to angiography guide the operator to focus on abnormal areas. Angiography generally is a safe procedure. The mortality rate for patients undergoing this procedure is less than 0.5%, and the morbidity rate is less than 5%. Patients who have long-standing pulmonary arterial hypertension and right ventricular failure are considered high-risk patients. Negative pulmonary angiogram findings, even if false negative, exclude clinically relevant pulmonary embolism.
If multidetector-row computed tomography angiography (MDCTA) is unavailable, conduct pulmonary angiography. Long the criterion standard for pulmonary embolism diagnosis, pulmonary angiography is nevertheless more invasive and harder to perform than MDCTA, and for these reasons, it is rapidly being replaced. However, pulmonary angiography remains a useful diagnostic modality when MDCTA cannot be performed.
When pulmonary angiography has been performed carefully and completely, a positive result provides virtually a 100% certainty that an obstruction to pulmonary arterial blood flow exists. A negative pulmonary angiogram provides a greater than 90% certainty for the exclusion of pulmonary embolism.
A positive angiogram is an acceptable endpoint no matter how abbreviated the study. However, a complete negative study requires the visualization of the entire pulmonary tree bilaterally. This is accomplished via selective cannulation of each branch of the pulmonary artery and injection of contrast material into each branch, with multiple views of each area. Even then, emboli in vessels smaller than third order or lobular arteries are not seen.
Small emboli cannot be seen angiographically, yet embolic obstruction of these smaller pulmonary vessels is very common when postmortem examination follows a negative angiogram. These small emboli can produce pleuritic chest pain and a small sterile effusion, even though the patient has a normal V/Q scan and a normal pulmonary angiogram.
In most patients, however, pulmonary embolism is a disease of multiple recurrences, with large and small emboli already present by the time the diagnosis is suspected. Under these circumstances, the V/Q scan and the angiogram are likely to detect at least some of the emboli.
Pulmonary angiography demonstrates subsegmental vessels in more detail than does CT scanning, although the superimposition of the small vessels remains a limiting factor. As a result, the interobserver agreement rate for isolated subsegmental pulmonary embolism is only 45%.
The routine use of CT pulmonary angiography for the detection of pulmonary emboli has led to overdiagnosis of the condition, according to a recent study. Over diagnosing pulmonary embolism has resulted in possible inappropriate treatment with anticoagulation, a leading cause of medication-related death.
Between 1998, when CT pulmonary angiography was introduced, and 2006, there was an 80% increase in the incidence of pulmonary embolism, but little subsequent drop in deaths, which suggests that many of the extra emboli being detected are not clinically important. During this period, the detection rate rose from 62.1 to 112.3 per 100,000 US adults and US deaths from pulmonary embolism dropped from 12.3 to 11.9 per 100,000.
Computed Tomography Scanning
Technical advances in CT scanning, including the development of multidetector-array scanners, have led to the emergence of CT scanning as an important diagnostic technique in suspected pulmonary embolism. Contrast-enhanced CT scanning is increasingly used as the initial radiologic study in the diagnosis of pulmonary embolism, especially in patients with abnormal chest radiographs in whom scintigraphy results are more likely to be nondiagnostic.
Computed tomography angiography (CTA) is the initial imaging modality of choice for stable patients with suspected pulmonary embolism. The American College of Radiology (ACR) considers chest CTA to be the current standard of care for the detection of pulmonary embolism. A study by Ward et al determined that a selective strategy in which CTA is used after compression ultrasonography is cost-effective for patients with a high pretest probability of pulmonary embolism. This strategy may reduce the need for the CTA and help eliminate adverse effects associated with CTA.
Toward a goal of reducing unnecessary CTA and associated radiation exposure, Drescher et al studied the effect of implementing a computerized decision support system for pulmonary embolism evaluation in the ED. Before implementation, the rate of positive pulmonary embolism diagnosis for CTAs performed was 8.3%; after, the positivity rate rose to 12.7%. The positive yield would have been higher (16.7%) had emergency physicians adhered in all cases to the outcome of the decision support system; in 27% of cases they did not.
Like pulmonary angiography, CT scanning shows emboli directly, but it is noninvasive, cheaper than pulmonary angiography, and widely available. CT scanning is the only test that can provide significant additional information related to alternate diagnoses; spiral (helical) CT scanning results may suggest an alternative diagnosis in up to 57% of patients. This is a clear advantage of CT scanning over pulmonary angiography or scintigraphy.
A study of multidetector computed tomography (MDCTA) for detection of right ventricular dysfunction in 457 patients with acute pulmonary embolism found a reasonable correlation with echocardiography, the reference standard. The criterion selected, a right-to-left ventricular dimensional ratio of 0.9 or more at MDCTA, had 92% sensitivity for right ventricular dysfunction. The combination of quantitative assessment of ventricular dimensions by CT and measurement of biomarkers may provide additional diagnostic accuracy for the presence of right ventricular dysfunction.
Chest Radiography
Chest radiographs are abnormal in most cases of pulmonary embolism, but the findings are nonspecific. Common radiographic abnormalities include atelectasis, pleural effusion, parenchymal opacities, and elevation of a hemidiaphragm. The classic radiographic findings of pulmonary infarction include a wedge-shaped, pleura-based triangular opacity with an apex pointing toward the hilus (Hampton hump) or decreased vascularity (Westermark sign). These findings are suggestive of pulmonary embolism but are infrequently observed.
The abrupt tapering or cutoff of a pulmonary artery secondary to an embolus (knuckle sign), cardiomegaly (especially on the right side of the heart), and pulmonary edema are other findings. In the appropriate clinical setting, these findings could be consistent with acute Cor pulmonale. A normal-appearing chest radiograph in a patient with severe dyspnea and hypoxemia, but without evidence of bronchospasm or a cardiac shunt, is strongly suggestive of pulmonary embolism.
The ACR recommends chest radiography as the most appropriate study for ruling out other causes of chest pain in patients with suspected pulmonary embolism. Initially, the chest radiographic findings are normal in most cases of pulmonary embolism. However, in later stages, radiographic signs may include a Westermark sign (dilatation of pulmonary vessels and a sharp cutoff), atelectasis, a small pleural effusion, and an elevated diaphragm. Generally, chest radiographs cannot be used to conclusively prove or exclude pulmonary embolism; however, radiography and electrocardiography may be useful for establishing alternative diagnoses.
Ventilation-Perfusion Scanning
V/Q scanning of the lungs is an important modality for establishing the diagnosis of pulmonary embolism. V/Q scanning may be used when CT scanning is not available or if the patient has a contraindication to CT scanning or intravenous contrast material. Children generally have a more homogenous perfusion scan; thus, deficits in perfusion are more likely to represent real or significant pulmonary embolism than they are in adults.
The PIOPED II trial provided high-, intermediate-, and low-probability criteria for V/Q scanning diagnosis of pulmonary embolism.
The high-probability criteria are as follows:
- Two large (>75% of a segment) segmental perfusion defects without corresponding ventilation or chest radiographic abnormalities
- One large segmental perfusion defect and two moderate (25-75% of a segment) segmental perfusion defects without corresponding ventilation or radiographic abnormalities
- Four moderate segmental perfusion defects without corresponding ventilation or chest radiographic abnormalities
- The intermediate-probability criteria are as follows:
- One moderate to less than two large segmental perfusion defects without corresponding ventilation or chest radiographic abnormalities
- Corresponding V/Q defects and radiographic parenchymal opacity in the lower lung zone
- Single moderate matched V/Q defects with normal chest radiograph findings
- Corresponding V/Q and chest radiography small pleural effusion
- Difficult to categorize as normal, low, or high probability
Low probability criteria are as follows:
- Multiple matched V/Q defects, regardless of size, with normal chest radiograph findings
- Corresponding V/Q defects and radiographic parenchymal opacity in upper or middle lung zone
- Corresponding V/Q defects and large pleural effusion
- Any perfusion defects with substantially larger radiographic abnormality
- Defects surrounded by normally perfused lung (stripe sign)
- More than three small (< 25% of a segment) segmental perfusion defects with normal chest radiograph findings
- Nonsegmental perfusion defects (cardiomegaly, aortic impression, enlarged hila)
- The normal finding is the presence of no perfusion defects and perfusion outlines the shape of the lung seen on a chest radiograph.
- The very low–probability criterion is the presence of three small (< 25% of a segment) segmental perfusion defects with normal chest radiograph findings.
- In the PIOPED II study, very low–probability V/Q scans in patients whose Wells score indicated low pretest probability of pulmonary embolism reliably excluded pulmonary embolism.
Electrocardiography
- The most common ECG abnormalities in the setting of pulmonary embolism are tachycardia and nonspecific ST-T wave abnormalities. The finding of S1 Q3 T3 is nonspecific and insensitive in the absence of clinical suspicion for pulmonary embolism. The classic findings of right heart strain and acute Cor pulmonale are tall, peaked P waves in lead II (P pulmonale); right axis deviation; right bundle-branch block; an S1 Q3T3 pattern; or atrial fibrillation. Unfortunately, only 20% of patients with proven pulmonary embolism have any of these classic electrocardiographic abnormalities. If electrocardiographic abnormalities are present, they may be suggestive of pulmonary embolism, but the absence of such abnormalities has no significant predictive value.
Magnetic Resonance Imaging
- With magnetic resonance imaging (MRI), evidence of pulmonary emboli may be detected by using standard or gated spin-echo techniques. Pulmonary emboli demonstrate increased signal intensity within the pulmonary artery. By obtaining a sequence of images, this signal that is originating from slow blood flow may be distinguished from pulmonary embolism. However, this remains a problem in pulmonary hypertension.
- Magnetic resonance angiography is performed following intravenous administration of gadolinium. Gadolinium-based contrast agents (gadopentetate dimeglumine [Magnevist], gadobenatedimeglumine [MultiHance], gadodiamide [Omniscan], gadoversetamide [OptiMARK], gadoteridol [ProHance]) have been linked to the development of nephrogenic systemic fibrosis (NSF) or nephrogenic fibrosing dermopathy (NFD). The disease has occurred in patients with moderate to end-stage renal disease after being given a gadolinium-based contrast agent to enhance MRI or magnetic resonance angiography scans.
- NSF/NFD is a debilitating and sometimes fatal disease. Characteristics include red or dark patches on the skin; burning, itching, swelling, hardening, and tightening of the skin; yellow spots on the whites of the eyes; joint stiffness with trouble moving or straightening the arms, hands, legs, or feet; pain deep in the hip bones or ribs; and muscle weakness.
- MRI has a sensitivity of 85% and specificity of 96% for central, lobar, and segmental emboli; MRI is inadequate for the diagnosis of subsegmental emboli.
- Few data are available regarding the use of MRI in children suspected of having a pulmonary embolism. Its use in these patients should be considered investigational at this time.
- Few investigators have reported the feasibility of MRI in the evaluation of pulmonary embolism. However, the role of MRI is mostly limited to the evaluation of patients who have impaired renal function or other contraindications for the use of iodinated contrast material. Newer blood-pool contrast agents and respiratory navigators may enhance the role of MRI in the diagnosis of pulmonary embolism.
Echocardiography
- This modality generally has limited accuracy in the diagnosis of pulmonary embolism. Transesophageal echocardiography may identify central pulmonary embolism, and the sensitivity for central pulmonary embolism is reported to be 82%. Overall sensitivity and specificity for central and peripheral pulmonary embolism is 59% and 77%.
- Echocardiography (ECHO) provides useful information. It may allow diagnosis of other conditions that may be confused with pulmonary embolism, such as pericardial effusion. ECHO allows visualization of the right ventricle and assessment of the pulmonary artery pressure. ECHO serves a prognostic function; the mortality rate is almost 10% in the presence of right ventricular dysfunction and 0% in the absence of right ventricular dysfunction. (Vanni et al reported that a right ventricular strain pattern is associated with a worse short-term outcome.) ECHO may be used to identify the presence of right-chamber emboli.
- The subcostal view is preferred at initial screening for mechanical activity and pericardial fluid and for gross assessment of global and regional abnormalities. To obtain a subcostal view, place the transducer on the left subcostal margin with the beam aimed at the left shoulder.
- The parasternal view allows visualization of the aortic valve, proximal ascending aorta, and posterior pericardium and permits determination of left ventricular size. It is particularly helpful when the subcostal view is difficult to obtain. To obtain a parasternal view, place the transducer in the left parasternal area between the second and fourth intercostal spaces. The plane of the beam is parallel to a line drawn from the right shoulder to the left hip.
Several echocardiographic findings have been proposed for noninvasive diagnosis of right ventricular dysfunction at the bedside, including right ventricular enlargement and/or hypokinesis of the free wall, leftward septal shift, and evidence of pulmonary hypertension. If right ventricular dysfunction is seen on cardiac ultrasonography, the diagnosis of acute sub massive or massive pulmonary embolism is supported. While the presence of right ventricular dysfunction can be used to support the clinical suspicion of pulmonary embolism, prognostic information can be obtained by assessing the severity of right ventricular dysfunction.
Duplex Ultrasonography
The diagnosis of pulmonary embolism can be proven by demonstrating the presence of a DVT at any site. This may sometimes be accomplished noninvasively by using duplex ultrasonography. To look for DVT using ultrasonography, the ultrasonographic transducer is placed against the skin and pressed inward firmly enough to compress the vein being examined. In an area of normal veins, the veins are easily compressed completely closed, while the muscular arteries are extremely resistant to compression. Where DVT is present, the veins do not collapse completely when pressure is applied using the ultrasonographic probe.
A prospective observational study of 146 patients with suspected or confirmed pulmonary embolism indicates that identification of right ventricular dilatation on bedside echocardiography may aid diagnosis of pulmonary embolism. Bedside echocardiography showed right ventricular dilatation in 15 of the 30 patients who had pulmonary emboli, compared with 2 of the 116 patients without pulmonary emboli.
The presence of right ventricular dilatation on bedside echocardiography had a sensitivity of 50%, specificity of 98%, and positive and negative predictive values of 88% for the diagnosis of pulmonary embolism. Most of the 15 patients with confirmed pulmonary emboli and right ventricular dilatation had proximal clots, while most of those with confirmed pulmonary emboli and a normal right ventricular/left ventricular ratio had more distal clots.
Note that a negative ultrasonographic scan does not rule out DVT, because many DVTs occur in areas that are inaccessible to ultrasonographic examination. Before an ultrasonographic scan can be considered negative, the entire deep venous system must be interrogated using centimeter-by-centimeter compression testing of every vessel. In two-thirds of patients with pulmonary embolism, the site of DVT cannot be visualized with ultrasonography, so a negative duplex ultrasonographic scan does not markedly reduce the likelihood of pulmonary embolism.
Approach Considerations
Even in patients who are fully anticoagulated DVT and pulmonary embolism (PE) can and often do recur. New PE in the hospital can occur in the following patients despite therapeutic anticoagulation:
- Patients who have nonfloating DVT without PE at presentation (3%)
- Patients who present with a floating thrombus, but have no PE (13%)
- Patients who present with PE but have no floating thrombus (11%)
- Patients who present with PE who have a floating thrombus visible at venography (39%)
Deciding how to treat a venous thrombosis that may lead to a PE is difficult. A survey of Canadian pediatric intensivists reported the following four patient factors commonly used to determine if a venous thrombosis is clinically important:
- Clinical suspicion of a PE
- Symptoms
- Detection of thrombosis on clinical examination
- Presence of an acute or chronic cardiopulmonary comorbidity that affects the patient’s ability to tolerate a PE
Thrombolysis for Pulmonary Embolism
All patients with PE require rapid risk stratification. Thrombolytic therapy should be used in patients with acute PE associated with hypotension (systolic BP< 90 mm HG) who do not have a high bleeding risk. Do not delay thrombolysis in this population because irreversible cardiogenic shock can develop. Thrombolytic therapy is suggested in select patients with acute PE not associated with hypotension and with a low bleeding risk whose initial clinical presentation or clinical course after starting anticoagulation suggests a high risk of developing hypotension. Assessment of pulmonary embolism severity, prognosis, and risk of bleeding dictate whether thrombolytic therapy should be started. Thrombolytic therapy is not recommended for most patients with acute PE not associated with hypotension.
Although most studies demonstrate the superiority of thrombolytic therapy with respect to the resolution of radiographic and hemodynamic abnormalities within the first 24 hours, this advantage disappears 7 days after treatment. Controlled clinical trials have not demonstrated benefits in terms of reduced mortality rates or earlier resolution of symptoms when currently compared with heparin. A large retrospective review suggests that the use of thrombolytic therapy in unstable patients with PE may lead to reduced mortality when compared to anticoagulation therapy alone. Concurrent use of thrombolytic therapy and vena cava filters in such patients may reduce mortality even further.
In a meta-analysis of 16 randomized studies comparing thrombolytic therapy with anticoagulation therapy in patients with pulmonary embolism, including intermediate-risk, hemodynamically stable patients with right ventricular dysfunction, Chatterjee et al found that thrombolytic therapy, as compared with standard anticoagulant therapy, reduced mortality by 47% but was associated with a 2.7-fold increase in major bleeding.
The investigators also found, however, that the rate of major bleeding was not significantly increased with thrombolysis among patients younger than 65 years, whereas it more than tripled in the subgroup of patients older than 65 years. Thrombolytic therapy was associated with a greater risk of intracranial hemorrhage and a lower risk of recurrent pulmonary embolism.
Embolectomy
Either catheter embolectomy and fragmentation or surgical embolectomy is reasonable for patients with massive pulmonary embolism who have contraindications to fibrinolysis or who remain unstable after receiving fibrinolysis. If these procedures are not available locally, it is reasonable to consider transferring the patient to an institution with experience in one of these procedures, providing the transfer can be accomplished safely.
In patients with sub-massive acute PE, either catheter embolectomy or surgical embolectomy may be considered if they have clinical evidence of an adverse prognosis (i.e., new hemodynamic instability, worsening respiratory failure, severe right ventricular dysfunction, or major myocardial necrosis). These interventions are not recommended for patients with low-risk or sub-massive acute pulmonary embolism who have minor right ventricular dysfunction, minor myocardial necrosis, and no clinical worsening.
Consultations
Fibrinolytic therapy should not be delayed while consultation is sought. The decision to treat pulmonary embolism by fibrinolysis is properly made by the responsible emergency physician alone, and fibrinolytic therapy is properly administered in the ED.
A pulmonologist is often consulted before the true diagnosis is made because of the nonspecific nature of the symptoms, and consultation with a cardiologist is warranted to rule out a cardiac etiology for the presenting symptoms and signs and to perform ECHO and pulmonary angiography.
If embolectomy is considered, consultation with a cardiac surgeon is mandatory. Few data are available regarding the use of surgical embolectomy in children. Consider embolectomy in the setting of massive cardiac failure when time is insufficient for natural or pharmacological thrombolysis or if thrombolysis is contraindicated. Thrombotic endarterectomy is another surgical treatment option for patients with hemodynamic compromise from large pulmonary emboli. Thrombotic endarterectomy is only performed at certain centers and has a high mortality rate, but it can be successful in certain populations.
A hematologist can suggest an appropriate work-up for a procoagulant defect and can recommend an anticoagulation regimen. Consultation with a hematologist is essential in children with sickle cell disease. A free clinical consultation service for complex cases of thromboembolism in children is available for clinicians by calling 1-800-NO-CLOTS (1-800-662-5687).
An interventional radiology consultation may be helpful for catheter-directed fibrinolysis in selected patients. In rare cases, arranging for the placement of a venous filter may be appropriate if the patient is not a candidate for thrombolytic therapy.
Vena Cava Filters
Patients with acute PE should not routinely receive vena cava filters in addition to anticoagulants. An ideal IVC filter should be easily and safely placed using a percutaneous technique, biocompatible and mechanically stable, and able to trap emboli without causing occlusion of the vena cava.
IVC interruption by the insertion of an IVC filter (Greenfield filter) is only indicated in the following settings:
- Patients with acute venous thromboembolism who have an absolute contraindication to anticoagulant therapy (e.g., recent surgery, hemorrhagic stroke, significant active or recent bleeding)
- Patients with massive PE who survived, but in whom recurrent embolism invariably will be fatal
- Patients who have objectively documented recurrent venous thromboembolism, adequate anticoagulant therapy notwithstanding
In patients with a time-limited indication for IVC filter placement (e.g., a short-term contraindication to anticoagulation), it is reasonable to select a retrievable IVC filter and evaluate the patient periodically for filter retrieval. After placement of an IVC filter, anticoagulation should be resumed once contraindications to anticoagulation or active bleeding complications have resolved.
Anticoagulation for Pulmonary Embolism
Unfractionated heparin therapy
In patients with acute PE, anticoagulation with IV UFH, LMWH, or fondaparinux is preferred over no anticoagulation. Most patients with acute PE should receive LMWH or fondaparinux instead of IV UFH. In patients with PE, if concerns regarding subcutaneous absorption arise, severe renal failure exists, or if thrombolytic therapy is being considered, IV UFH is the recommended form of initial anticoagulation. Clinicians often choose to use IV UFH in preference to LMWH and fondaparinux in specific clinical circumstances where medical or surgical procedures are likely to be performed and the short half-life of IV UFH allows for temporary cessation of anticoagulation and presumed reduction of bleeding risk during the procedure. Though this strategy has limited supporting evidence, it appears to represent a reasonable practice.
The efficacy of heparin therapy depends on achieving a critical therapeutic level of heparin within the first 24 hours of treatment. The critical therapeutic level of heparin is 1.5 times the baseline control value or the upper limit of the normal range of the activated partial thromboplastin time (aPTT).
This level of anticoagulation is expected to correspond to a heparin blood level of 0.2-0.4 U/ml by the protamine sulfate titration assay and 0.3-0.6 by the anti-factor X assay.
Each laboratory should establish the minimal therapeutic level for heparin, as measured by the aPTT, to coincide with a heparin blood level of at least 0.2 U/ml for each batch of thromboplastin reagent being used.
If IV UFH is chosen, an initial bolus of 80 U/kg or 5000 U followed by an infusion of 18 U/kg/h or 1300 U/h should be given, with the goal of rapidly achieving and maintaining the aPTT at levels that correspond to therapeutic heparin levels. Fixed-dose and monitored regimens of subcutaneous UFH are available and are acceptable alternatives.
Low-molecular-weight heparin therapy
Current guidelines for patients with acute PE recommend LMWH over IV UFH (grade 2C) and over SC UFH (grade 2B).In patients being treated with LMWH, once-daily regimens are preferred over twice-daily regimens (grade 2C). The choice between fondaparinux and LMWH should be based on local considerations to include cost, availability, and familiarity of use.
LMWHs have many advantages over UFH. These agents have a greater bioavailability, can be administered by subcutaneous injections, and have a longer duration of anticoagulant effect. A fixed dose of LMWH can be used, and laboratory monitoring of aPTT is not necessary.
Trials comparing LMWH with UFH have shown that LMWH is at least as effective and as safe as UFH. The studies have not pointed to any significant differences in recurrent thromboembolic events, major bleeding, or mortality between the 2 types of heparin.
LMWH can be administered safely in an outpatient setting. This has led to the development of programs in which clinically stable patients with PE are treated at home, at substantial cost savings. The ACCP guidelines suggest that patients with low-risk PE and who have acceptable home circumstances be discharged early from the hospital (i.e., before the first five days of treatment)(grade 2B).
An international, open-label, randomized trial compared outpatient and inpatient treatment (both using the LMWH enoxaparin as initial therapy) of low-risk patients with acute PE and concluded that outpatient treatment was noninferior to inpatient treatment.
Direct thrombin inhibitors and factor Xa inhibitors
Apixaban, dabigatran, rivaroxaban, and edoxaban are alternatives to warfarin for prophylaxis and treatment of PE. Apixaban, edoxaban, and rivaroxaban inhibit factor Xa, whereas dabigatran is a direct thrombin inhibitor.
Rivaroxaban
Rivaroxaban (Xarelto) is an oral factor Xa inhibitor approved by the FDA in November 2012 for the treatment of DVT or PE, and to reduce the risk of recurrent DVT and PE following initial treatment.
Approval for this indication was based on studies totaling 9478 patients with DVT or PE. Participants were randomly assigned to receive rivaroxaban, a combination of enoxaparin and a vitamin K antagonist (VKA) (e.g., warfarin), or a placebo. Study endpoints were designed to measure the number of patients who experienced recurrent symptoms of DVT, PE, or death after receiving treatment. Additionally, results from extended treatment demonstrated a reduced risk of recurrent DVT and PE. Approximately 1.3% in the rivaroxaban group experienced recurrent DVT or PE compared with 7.1% in the placebo group.
The results of the Einstein-PE study provide an important advance in the treatment of symptomatic PE. In a prospective, open-label study, 4832 patients were randomized to receive either rivaroxaban or enoxaparin followed by an adjusted-dose vitamin K antagonist for 3, 6, or 12 months. Treatment with a fixed-dose regimen of rivaroxaban was noninferior to standard therapy and had a satisfactory safety profile.
Data from a pooled analysis of the EINSTEIN-PE and EINSTEIN-DVT studies in the treatment of DVT or pulmonary embolism suggest that rivaroxaban is as effective in preventing VTE recurrence as the administration of enoxaparin followed by a vitamin-K antagonist. Rivaroxaban may also be associated with less bleeding, particularly in elderly patients and those with moderate renal impairment.
Apixaban
Apixaban was approved for the treatment of PE in August 2014. The approval for treatment of PE and prevention of recurrence was based on the outcome of the AMPLIFY (Apixaban for the Initial Management of Pulmonary Embolism and Deep-Vein Thrombosis as First-Line Therapy) and AMPLIFY-EXT studies, in which apixaban therapy was compared with enoxaparin and warfarin treatment. The AMPLIFY study showed that in comparison with the standard anticoagulant regimen apixaban therapy resulted in a 16% reduction in the risk of a composite endpoint that included recurrent symptomatic venous thromboembolism (VTE) or VTE-associated death.
This advance offers the prospect of a safe and effective regimen of anticoagulation for patients with the advantages of simplicity and cost-effectiveness in comparison to current management strategies.
Dabigatran
Dabigatran (Pradaxa) was approved by the FDA in 2014 for the treatment of DVT and PE and reducing venous thromboembolic recurrence. In the RE-COVER and RE-COVER 2 studies, patients with DVT and PE who had received initial parenteral anticoagulation (e.g., IV heparin, SC LMWH) for 5-10 days were randomized to warfarin or dabigatran. These two trials showed dabigatran was noninferior to warfarin in reducing DVT and PE and was associated with lower bleeding rates.
Edoxaban
Edoxaban (Savaysa) was approved by the FDA in January 2015 for the treatment of DVT and PE in patients who have been initially treated with a parenteral anticoagulant for 5-10 days. Approval was based on the Hokusai-VTE study, which included 3,319 patients with PE. Of these patients, 938 had right ventricular dysfunction, as assessed by measurement of N-terminal pro-brain natriuretic peptide levels. The rate of recurrent VTE in this subgroup was 3.3% in the edoxaban group and 6.2% in the warfarin group. Edoxaban was noninferior to high-quality standard warfarin therapy and caused significantly less bleeding in a broad spectrum of patients with VTE, including those with severe pulmonary embolism.
Betrixaban
Betrixaban, a factor Xa inhibitor, was approved by the FDA in June 2017. It is indicated for prophylaxis of VTE in adults hospitalized for acute medical illness who are at risk for thromboembolic complications owing to moderate or severe restricted mobility and other risk factors that may cause VTE.
Approval of betrixaban was based on data from the phase 3 APEX studies. These randomized, double-blind, multinational clinical trials compared extended-duration betrixaban (35-42 days) to short-duration enoxaparin (6-14 days) for VTE in 7513 acutely medically ill hospitalized patients with VTE risk factors.
Patients in the betrixaban group took an initial dose of 160 mg orally on day 1, followed by 80 mg once daily for 35-42 days, and received a placebo injection once daily for 6-14 days. Patients in the enoxaparin group received 40 mg subcutaneously once daily for 6-4 days and took an oral placebo once daily for 35-42 days.
Efficacy was measured in 7441 patients using a composite outcome score composed of the occurrence of asymptomatic or symptomatic proximal deep vein thrombosis, nonfatal pulmonary embolism, stroke, or VTE-related death. Betrixaban showed significant decreases in VTE events compared with enoxaparin.
Fondaparinux
In patients with acute PE, fondaparinux as initial treatment is favored over IV UFH and over SC UFH. The choice between fondaparinux and LMWH should be based on local considerations to include cost, availability, and familiarity of use. Fondaparinux is a synthetic polysaccharide derived from the antithrombin binding region of heparin. Fondaparinux catalyzes factor Xa inactivation by antithrombin without inhibiting thrombin.
Once-daily fondaparinux was found to have similar rates of recurrent PE, bleeding, and death as IV UFH, according to one randomized, open-label study of 2213 patients with symptomatic pulmonary embolism.
In general, the use of LMWH or fondaparinux is recommended over IV UFH and SC UFH. This is because of a more predictable bioavailability, more rapid onset of full anticoagulant effect, and the benefit of not typically needing to monitor anticoagulant effect. However, if uncertainty arises regarding the accuracy of dosing, factor Xa levels can be monitored to determine efficacy.
Warfarin therapy
A vitamin K antagonist such as warfarin should be started on the same day as anticoagulant therapy in patients with acute PE. Parenteral anticoagulation and warfarin should be continued together for a minimum of at least five days and until the INR is 2.0.
The anticoagulant effect of warfarin is mediated by the inhibition of vitamin K–dependent factors, which are II, VII, IX, and X. The peak effect does not occur until 36-72 hours after drug administration, and the dosage is difficult to titrate.
A prothrombin time ratio is expressed as an INR and is monitored to assess the adequacy of warfarin therapy. The recommended therapeutic range for venous thromboembolism is an INR of 2-3. This level of anticoagulation markedly reduces the risk of bleeding without the loss of effectiveness. Initially, INR measurements are performed on a daily basis; once the patient is stabilized on a specific dose of warfarin, the INR determinations may be performed every 1-2 weeks or at longer intervals.
Duration of anticoagulation therapy
A patient with a first thromboembolic event occurring in the setting of reversible risk factors, such as immobilization, surgery, or trauma, should receive warfarin therapy for at least 3 months. No difference in the rate of recurrence was observed in either of 2 studies comparing 3 versus 6 months of anticoagulant therapy in patients with idiopathic (or unprovoked) first events. The current recommendation is anticoagulation for at least 3 months in these patients; the need for extending the duration of anticoagulation should be reevaluated at that time.
The current ACCP guidelines recommend that all patients with unprovoked PE receive three months of treatment with anticoagulation over a shorter duration of treatment and have an assessment of the risk-benefit ratio of extended therapy at the end of three months (grade 1B). Patients with the first episode of venous thromboembolism and with a low or moderate risk of bleeding should have extended anticoagulant therapy (grade 2B). Patients with a first episode of venous thromboembolism who have a high bleeding risk should have therapy limited to three months (grade 1B).
In patients with a second unprovoked episode of venous thromboembolism and low or moderate risk of bleeding, extended anticoagulant therapy is recommended (grades 1B and 2B, respectively). In patients with a second episode of venous thromboembolism and a high risk of bleeding, three months of anticoagulation are preferred overextended anticoagulation (grade 2B).
Patients who have PE and preexisting irreversible risk factors, such as deficiency of antithrombin III, protein S and C, factor V Leiden mutation, or the presence of antiphospholipid antibodies, should be placed on long-term anticoagulation.
Cancer patients
Patients who have PE in association with an active neoplasm provide challenges for long-term management because of their increased continuing risk for recurrent VTE and PE. The ninth edition of the ACCP guidelines recommends that such patients receive extended anticoagulation as opposed to three-month therapy if they are at low or moderate risk of bleeding complications (grade 1B). If patients with active neoplasm are at high risk of bleeding, it is still suggested that they receive extended therapy, though the supporting evidence is less conclusive (grade 2B). For the treatment of PE in cancer patients, LMWH is recommended in preference to a vitamin K antagonist such as warfarin (grade 2B). However, some cancer patients choose not to have long-term treatment with LMWH because of the need for daily injections and treatment costs. If cancer patients with PE choose not to have treatment with LMWH, a vitamin K antagonist such as warfarin is preferred over dabigatran or rivaroxaban (grade 2C).
Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia (HIT) is a transient prothrombotic disorder initiated by heparin. The main features of HIT are (1) thrombocytopenia resulting from immunoglobulin G–mediated platelet activation and (2) in vivo thrombin generation and increased risk of venous and arterial thrombosis.
The highest frequency of HIT, 5%, has been reported in post–orthopedic surgery patients receiving up to 2 weeks of unfractionated heparin. HIT occurred in approximately 0.5% of post–orthopedic surgery patients receiving LMWH for up to 2 weeks.
HIT may manifest clinically as an extension of the thrombus or formation of new arterial thrombosis. HIT should be suspected whenever the patient’s platelet count falls to less than 100,000/µL or less than 50% of the baseline value, generally after 5-15 days of heparin therapy. For patients receiving heparin where the risk of HIT is thought to be greater than 1%, guidelines suggest that platelet counts be obtained every two or three days from day 4 to day 14 of therapy, or until the heparin is stopped (grade 2C). The definitive diagnosis is made by performing a platelet activation factor assay.
The treatment of patients who develop HIT is to stop all heparin products, including catheter flushes and heparin-coated catheters, and to initiate an alternative, non-heparin anticoagulant, even when thrombosis is not clinically apparent. In patients with HIT with or without thrombosis, the use of lepirudin, argatroban, or danaparoid is preferred over the continued use of heparin, LMWH, or either initiation or continuation of a vitamin K antagonist (grade 1C).
If a vitamin K antagonist has already been started when HIT is diagnosed, guidelines recommend that it be discontinued and that vitamin K should be administered (Grade 2C). When HIT has been confirmed, vitamin K antagonists should not be started until the platelet count has recovered to at least 150 x 109/L (grade 1C), it should be started at low doses (i.e., 5 mg of warfarin), and it should be given concomitantly with a non-heparin anticoagulant for a minimum of five days and until the INR is within the target range (grade 1C).In patients with renal failure who have HIT and thrombosis, argatroban is preferred over other non-heparin anticoagulants (grade 2C).
Resistance to heparin
Few patients with venous thromboembolism require large doses of heparin for achieving an optimal activated partial thromboplastin time (aPTT). Those patients who do require them to have increased plasma concentrations of factor VIII and heparin-binding proteins. Increased factor VIII concentration causes dissociation between aPTT and plasma heparin values. The aPTT is suboptimal, but patients have adequate heparin levels upon protamine titration. This commonly occurs in patients with a concomitant inflammatory disease.
Monitoring the antifactorXa assay results in this situation is safe, effective and results in a less escalation of the heparin dose when compared with monitoring with aPTT. Whenever a therapeutic level of aPTT cannot be achieved with large doses of UFH, either the determination of plasma heparin concentration or therapy with LMWH should be instituted.
Supportive Care
Compression stockings
For patients who have had a proximal DVT, the use of elastic compression stockings provides a safe and effective adjunctive treatment that can limit postphlebitic syndrome. Stockings with a pressure of 30-40 mm Hg at the ankle, worn for 2 years following diagnosis, are recommended (grade 2B) to reduce the risk of the postphlebitic syndrome.
True gradient compression stockings are highly elastic, providing a gradient of compression that is highest at the toes and gradually decreases to the level of the thigh. This reduces capacitive venous volume by approximately 70% and increases the measured velocity of blood flow in the deep veins by a factor of 5 or more. Compression stockings of this type have been proven effective in the prophylaxis of thromboembolism and are also effective in preventing the progression of a blood clot in patients who already have DVT and pulmonary embolism.
A 1994 meta-analysis calculated a DVT risk odds ratio of 0.28 for gradient compression stockings (as compared to no prophylaxis) in patients undergoing abdominal surgery, gynecologic surgery, or neurosurgery.
Other studies found that gradient compression stockings and LMWH were the most effective modalities in reducing the incidence of DVT after hip surgery; they were more effective than subcutaneous UFH, oral warfarin, dextran, or aspirin.
The ubiquitous white stockings known as anti-embolic stockings or “Ted hose” produce a maximum compression of 18 mm Hg. Ted hose rarely are fitted in such a way as to provide even that inadequate gradient compression. Because they provide such limited compression, they have no efficacy in the treatment of DVT and pulmonary embolism, nor have they been proven effective as a prophylaxis against a recurrence.
True 30-40 mm Hg gradient compression pantyhose are available in sizes for pregnant women. They are recommended by many specialists for all pregnant women because they not only prevent DVT, but they also reduce or prevent the development of varicose veins during pregnancy.
Additional support therapies
Activity is recommended as tolerated. Early ambulation is recommended over bed rest when feasible (grade 2C recommendation).
Pharmacological support of the cardiovascular system may be necessary. Dopamine and dobutamine are the usual inotropic agents. Mechanical ventilation may be necessary to provide respiratory support and as adjunctive therapy for a failing circulatory system.
Children with sickle cell disease who present with pulmonary symptoms require treatment with a macrolide and cephalosporin antibiotic. Their clinical status should be closely monitored in order to anticipate those children who may develop an acute chest syndrome. Transfusion with packed red blood cells (either simple or exchange) improves oxygenation immediately, helping to break the vicious cycle outlined above.
IV fluids may help or may hurt the patient who is hypotensive from pulmonary embolism, depending on which point on the Starling curve describes the patient’s condition. A cautious trial of a small fluid bolus may be attempted, with careful surveillance of the systolic and diastolic blood pressures and immediate cessation if the situation worsens after the fluid bolus. Improvement or normalization of blood pressure after fluid loading does not mean the patient has become hemodynamically stable.
Individuals with acute, submassive pulmonary embolisms have low levels of endogenous activated protein C. A study by Dempfle et al. determined that administering drotrecogin alfa (activated) along with therapeutic doses of enoxaparin enhanced the inhibition of fibrin formation in these patients.
DrotrecoginAlfa was withdrawn from the worldwide market October 25, 2011 after analysis of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS)-SHOCK clinical trial. DrotrecoginAlfa failed to demonstrate a statistically significant reduction in 28-day all-cause mortality in patients with severe sepsis and septic shock. Trial results observed a 28-day all-cause mortality rate of 26.4% in patients treated with activated DrotrecoginAlfa compared with 24.2% in the placebo group of the study.
Long-Term Monitoring
PT should be measured on a regular basis; the goal is an INR of 2-3.
The length of treatment depends on the presence of risk factors. If no underlying risk factors are present, therapy can be stopped within 1-2 months. If risk factors are present, especially anticardiolipin antibodies, therapy should continue for at least 4-6 months.
Long-term anticoagulation is essential for patients who survive an initial DVT or pulmonary embolism. The optimum total duration of anticoagulation is controversial, but general consensus holds that at least 6 months of anticoagulation are associated with significant reduction in recurrences and a net positive benefit.
Patients may have treatment initiated using concomitant warfarin and unfractionated heparin for 5 days in the hospital, with discharge on warfarin alone when the international normalized ratio (INR) is 2. Alternatively, patients may be discharged on concomitant therapy with a LMWH and warfarin for at least 5 days. The LMWH is then discontinued in the outpatient setting when the INR reaches 2.
Pulmonary Embolism in Pregnancy
The risk of venous thromboembolism is increased during pregnancy and the postpartum period. Pulmonary embolism is the leading cause of death in pregnancy. DVT and pulmonary embolism are common during all trimesters of pregnancy and for 6-12 weeks after delivery.
Diagnosis
The diagnostic approach to patients with pulmonary embolism should be exactly the same in a pregnant patient as in a nonpregnant one. A nuclear perfusion lung scan is safe in pregnancy, as is a chest CT scan.
Guidelines by the professional societies on the diagnosis of pulmonary embolism make this difficult assessment easier and reduce the risks of radiation to the fetus. If the patient has a low pretest probability for pulmonary embolism and a normal D-dimer test result, clinical exclusion from further investigations is recommended. When the suspicion is high, the patients should have bilateral leg Doppler assessment. If the results are positive, the patient should be treated for pulmonary embolism. If the results are negative, CT pulmonary angiography is the next step. To rule out contrast-induced hypothyroidism, all neonates exposed to the iodinated contrast in utero should have their serum thyrotropin level checked in the first week of life.
Treatment
Heparin and fibrinolysis are safe in pregnancy. Failure to treat the mother properly is the most common cause of fetal demise.
Pregnant patients diagnosed with DVT or pulmonary embolism may be treated with LMWH throughout their pregnancy. Warfarin is contraindicated, because it crosses the placental barrier and can cause fetal malformations. Unfractionated heparin is category C. Therefore, LMWH at full anticoagulation doses should be continued until delivery. Women experiencing a thromboembolic event during pregnancy should receive therapeutic treatment with unfractionated heparin or LMWH during pregnancy, with anticoagulation continuing for 4-6 weeks postpartum and for a total of at least 6 months.
In addition to the thrombotic risks in pregnancy, women of childbearing age who are prescribed warfarin should be advised of the teratogenic effects of this drug. Alteplase is a category C drug and should only be given following a judicious assessment of the risk-to-benefit ratio.
Pregnant women who are in a hypercoagulable state or who have had previous venous thromboembolism should receive prophylactic anticoagulation during pregnancy.
Prevention
Preventing idiopathic outpatient pulmonary embolism is difficult, if not impossible. That said, the majority of pulmonary embolisms occur in hospitalized patients. The incidence in these cases can be reduced through appropriate prophylaxis, achieved mechanically or via the administration of heparin, LMWH, or warfarin.
The incidence of venous thrombosis, pulmonary embolism, and death can be significantly reduced by embracing a prophylactic strategy in high-risk patients. Prevention of DVT in the lower extremities inevitably reduces the frequency of pulmonary embolism; therefore, populations at risk must be identified, and safe and efficacious prophylactic modalities should be used.
Regular physical exercise, leg elevation, and wearing compression stockings may aid in preventing pulmonary embolism.
The QThrombosis algorithm is intended to identify currently asymptomatic adults at greatest future risk of venous thrombosis based on established risk factors. According to the study in which it was developed and validated, QThrombosis estimates the absolute risk of venous thrombosis at 1 year and 5 years into the future, information that can be used to guide prophylaxis and medication decisions.
Complications
Complications of pulmonary embolism include the following:
- Sudden cardiac death
- Obstructive shock
- Pulseless electrical activity
- Atrial or ventricular arrhythmias
- Secondary pulmonary arterial hypertension
- Cor pulmonale
- Severe hypoxemia
- Right-to-left intracardiac shunt
- Lung infarction
- Pleural effusion
- Paradoxical embolism
- Heparin-induced thrombocytopenia
- Thrombophlebitis
Prognosis
The prognosis of patients with pulmonary embolism depends on two factors: the underlying disease state and appropriate diagnosis and treatment. Approximately ten percent of patients who develop a pulmonary embolism die within the first hour and thirty percent dies subsequently from recurrent embolism. Mortality for acute pulmonary embolism can be broken down into two categories – massive pulmonary embolism and non-massive pulmonary embolism.
Anticoagulant treatment decreases mortality to less than five percent. At five days of anticoagulant therapy, thirty-six percent of lung scan defects are resolved; at two weeks, fifty-two percent is resolved; and at three months, seventy-three percent is resolved. Most patients treated with anticoagulants do not develop long-term sequelae upon follow-up evaluation. The mortality in patients with undiagnosed pulmonary embolism is thirty percent.
In the PIOPED study, the 1-year mortality rate was 24%. The deaths occurred due to cardiac disease, recurrent pulmonary embolism, infection, and cancer.
The risk of recurrent pulmonary embolism is due to the recurrence of proximal venous thrombosis; approximately 17% of patients with recurrent pulmonary embolism were found to have proximal DVT. In a small proportion of patients, pulmonary embolism does not resolve; hence, chronic thromboembolic pulmonary arterial hypertension results.
Elevated plasma levels of Natriuretic peptides (brain Natriuretic peptide and N -terminal pro-brain natriuretic peptide) have been associated with higher mortality in patients with pulmonary embolism. In one study, levels of N -terminal pro-brain Natriuretic peptide greater than 500 ng/L were independently associated with central pulmonary embolism and were a possible predictor of death from a pulmonary embolism.
In a study of 270 adult patients with symptomatic pulmonary embolism that was objectively confirmed, researchers found that elevated plasma lactate levels (≥2 mmol/L) were associated with an increased risk of mortality and other adverse outcomes, independent of shock, hypotension, right-sided ventricular dysfunction, or injury markers.
Massive pulmonary embolism
As a cause of sudden death, massive pulmonary embolism is second only to sudden cardiac death. Massive pulmonary embolism is defined as presenting with a systolic arterial pressure less than 90 mm Hg. The mortality for patients with massive pulmonary embolism is between 30% and 60%, depending on the study cited. Autopsy studies of patients who died unexpectedly in a hospital setting have shown approximately 80% of these patients died from a massive pulmonary embolism.
Most deaths from massive pulmonary embolism occur in the first 1-2 hours of care, so it is important for the initial treating physician to have a systemized, aggressive evaluation and treatment plan for patients presenting with pulmonary embolism.
Non-massive pulmonary embolism
Non-massive pulmonary embolism is defined as having a systolic arterial pressure greater than or equal to 90 mm Hg. This is the most common presentation for pulmonary embolism and accounts for 95.5-96% of the patients.
Hemodynamically stable pulmonary embolism has a much lower mortality rate because of treatment with anticoagulant therapy. In non-massive pulmonary embolism, the death rate is less than 5% in the first 3-6 months of anticoagulant treatment. The rate of recurrent thromboembolism is less than 5% during this time. However, recurrent thromboembolism reaches 30% after 10 years.
Patient Education
The importance of adherence to the treatment regimen should be repeatedly stressed. The patient should be instructed regarding what to do in the event of any bleeding complications. Because most patients are administered warfarin or low molecular weight heparin upon discharge from the hospital, they must be advised regarding potential interactions between these agents and other medications.

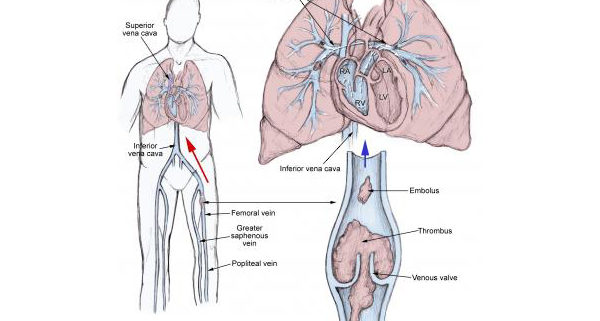
Leave a Reply
Want to join the discussion?Feel free to contribute!