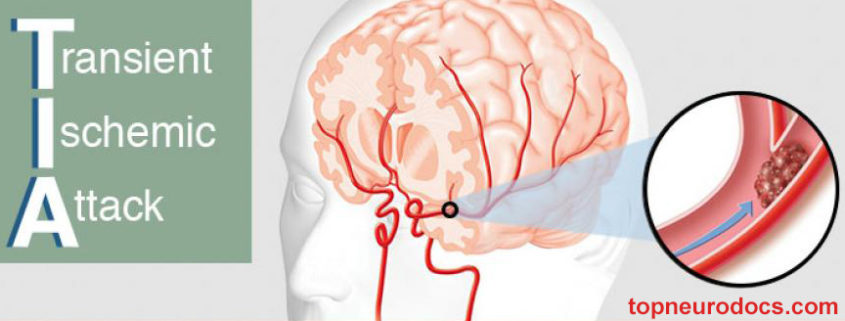
Transient Ischemic Attack (TIA)
A transient ischemic attack (TIA) is an acute episode of temporary neurologic dysfunction that results from focal cerebral, spinal cord, or retinal ischemia, and is not associated with acute tissue infarction.
TIAs are often labeled “mini-strokes,” because they can be relatively benign in terms of immediate consequences. The term “warning stroke” is more appropriate for these temporary episodes, because a TIA can signal a problem that may lead to further strokes, disability, or even death.
Even though TIA symptoms usually last less than five minutes with an average of about a minute, with no noticeable or lasting effects, anyone who has symptoms should be rushed to the emergency room.
When stroke symptoms are first noticed, it isn’t safe to assume they will disappear without urgent medical care. Trained medical staff should evaluate the patient’s condition. Some signs are only visible with hospital equipment, so appropriate medical care is important.
Patient’s History
The clinical symptoms of TIA typically last less than 1 hour and often last for less than 30 minutes, but prolonged episodes can occur. Symptoms often resolve before the patient presents to a clinician. Thus, historical questions should be addressed not just to the patient but also to family members, witnesses, and emergency medical services (EMS) personnel.
Witnesses often perceive abnormalities that the patient cannot, such as changes in behavior, speech, gait, memory, and movement. The family or witnesses should be instructed to go to the emergency department (ED), or contact information for these individuals should be obtained.
These various resources should be used to clarify when symptoms first occurred, how long they lasted, whether the patient recovered completely (i.e., returned to baseline status), and if a pattern of escalating symptoms is present. For patients who woke up or are found with symptoms, the time they were last known to be normal should be documented.
Patients with possible transient ischemic attack (TIA) require urgent evaluation and risk stratification. Rapid transport to hospital is essential to evaluate the patient who may have fleeting or stuttering symptoms. In some communities, emergency medical services (EMS) may preferentially transfer patients with high-risk stroke symptoms to centers with specific stroke expertise.
Significant medical history questions to elicit any risk factors for relevant underlying disease include questions about the following:
- Recent surgery (e.g., carotid or cardiac)
- Previous strokes or TIAs
- Seizures
- Systemic or central nervous system (CNS) infections
- Use of illicit drugs
- Complete medication regimen, including all over-the-counter medications
- Comorbidities related to metabolic disorders, especially diabetes
- Known coagulopathy or family history of early clotting or thrombotic events
- History of arteritis
- Noninfectious necrotizing vasculitis, irradiation, and local trauma
- Thromboembolic risk factors (e.g., carotid artery stenosis, venous or arterial thromboembolism, patent foramen ovale or atrial septal defect, atrial fibrillation, prior myocardial infarction, and left ventricular dysfunction)
- Other known cardiovascular disease
- History of migraine
If a patient has a history of associated trauma or cardiac symptoms, the differential diagnosis widens. Pertinent negative items (e.g., lack of headache, lack of chest pain, and lack of eye pain) in the review of systems are also important.
Carotid or vertebral dissection can occur in association with both major and minor trauma. The patient may provide a history of blunt or torsion injury to the neck. An apparent association between cervical manipulation (as in chiropractic neck adjustment or massage therapy) and arterial dissections has been frequently reported.
Reviewing the patient’s medical record is extremely important for identifying deficits from previous strokes, seizures, or cardiac events. The primary care physician can be a reliable resource for insights into previous episodes and workup.
Initiation of stroke prevention therapy must be provided urgently. Medical management is aimed at reducing both short-term and long-term risk of stroke and varies according to the underlying cause of the episode.
Initial Assessment
Initial vital signs should include the following:
- Temperature
- Blood pressure
- Heart rate and rhythm
- Respiratory rate and pattern
- Oxygen saturation
Vital signs must be obtained promptly and addressed as indicated. A 12-lead electrocardiogram (ECG) with rhythm strip should be obtained and evaluated for symptomatic arrhythmias or evidence of ischemia. Cardiac monitoring can capture a relevant dysrhythmia. Pulse oximetry can evaluate for hypoxia.
The examiner should assess the patient’s overall health and appearance, making an assessment of the following:
- Attentiveness
- Ability to interact with the examiner
- Language and memory skills
- Overall hydration status
- Development
Initial assessment is aimed at excluding emergency conditions that can mimic a TIA, which include the following:
- Hypoglycemia
- Seizure
- Intracranial hemorrhage
Fingerstick glucose testing can quickly rule out hypoglycemia. IV access (if not already established by EMS) should be obtained and blood drawn for a complete blood count (CBC), coagulation studies, and serum electrolyte levels. Transport should not be delayed to obtain IV access.
The goals of the physical examination are to uncover any neurologic deficits, to evaluate for underlying cardiovascular risk factors, and to seek any potential thrombotic or embolic source of the event.
Identify signs of other active comorbidities, including infections (e.g., sinusitis, mastoiditis, and meningitis) and vasculitides. The carotid arteries can be examined for pulse upstroke or bruit, and the neck can be examined for the presence of carotid endarterectomy scars.
Funduscopy can identify retinal plaques, retinal pigmentation, and optic disc margins. Pupil reaction to direct and consensual light exposure can be assessed.
In addition to performing standard auscultation, examine the chest for the presence of surgical scars, for the presence of a pacemaker or automatic implantable cardioverter-defibrillator (ICD), or for other clues that the patient may have a cardiac disorder and may be increased risk for a cardioembolic phenomenon.
Cardioembolic events are significant causes of TIAs. Assess for irregular rhythm or other unusual rhythms and rates, murmurs, or rubs that might suggest valvular disease, atrial-septal defects, or ventricular aneurysm (a source of mural thrombi). Check for splinter hemorrhages in the nail beds.
Neurologic Examination
A neurologic examination is the foundation of the TIA evaluation and should focus in particular on the neurovascular distribution suggested by the patient’s symptoms. Subsets of the neurologic examination include the following:
- Cranial nerve testing
- Determination of somatic motor strength
- Somatic sensory testing
- Speech and language testing
- Assessment of the cerebellar system (be sure to watch the patient walk)
For somatic motor testing, test muscle stretch reflexes of the biceps, triceps, brachioradialis, patellar, and Achilles. In addition, inspect posture and look for tremors. Test the strength of the shoulder girdle, upper extremities, abdominal muscles, and lower extremities. Test passive movement of major joints to look for spasticity, clonus, and rigidity.
Cranial Nerves
The following signs may be present in patients with cranial nerve dysfunction:
- Ocular dysmotility
- Forehead wrinkling asymmetry
- Incomplete eyelid closure
- Asymmetrical mouth retraction
- Loss of the nasolabial crease
- Swallowing difficulty
- Lateral tongue movement
- Weak shoulder shrugging
- Visual field deficits
The cerebellar system can be tested by assessing ocular movement, gait, and finger-to-nose and heel-to-knee movements, with an eye to signs of past-pointing and dystaxia, hypotonia, overshooting, gait dystaxia, and nystagmus. The speech and language system can be tested to assess for both aphasia and dysarthria. Mental status can be assessed formally (e.g., with the Mini-Mental Status Examination or Quick Confusion Scale) or as part of the patient’s overall response to questions and interactions with the examiner.
Ideally, any neurologic deficits should be recorded with the aid of a formal and reproducible stroke scale, such as the National Institutes of Health Stroke Scale (NIHSS). A stroke scale prompts the examiner to be thorough and allows different examiners to repeat the examination reliably during subsequent phases of the evaluation. Any neurologic abnormalities should suggest the diagnosis of stroke (or ongoing neurologic event) rather than TIA.
Global CNS depression and airway or cardiac compromise are not typically features of a TIA. In fact, the level of consciousness and neurologic examination findings are expected to be at the patient’s baseline.
Imaging Studies
Brain imaging is recommended within 24 hours of symptom onset. Although magnetic resonance imaging (MRI) with diffusion-weighted imaging (DWI) is preferred, non-contrast computed tomography (CT) of the head is a reasonable first choice when MRI is not readily available.
The cerebral vasculature should be imaged on an urgent basis, preferably at the same time as the brain. Brain imaging can identify an area of ischemia in as many as 25% of patients, and TIA mimics may be identified as well. Vessel imaging can identify a stenosis or occlusion that may warrant early intervention.
Non-contrast Cranial Computed Tomography
Non-contrast cranial CT is widely and rapidly available and often serves as the initial imaging evaluation. It can aid in diagnosing the following:
- A new area of ischemia or infarction
- Old areas of ischemia
- Intracranial mass, such as tumor
- Intracranial bleeding, such as subdural hematoma or intracerebral hemorrhage
Magnetic Resonance Imaging
MRI is more sensitive than CT for acute ischemia, infarction, previous intracranial bleeding, and other underlying lesions; however, it is less widely available on an acute basis than CT.
The presence of ischemic lesions on MRI appears to increase the short-term risk of stroke, a finding that highlights the value of this modality in acute risk stratification. In addition, negative DWI in concert with low-risk clinical features can identify patients at minimal short-term stroke risk. Patients with DWI abnormalities, despite low ABCD2 scores, may be at just as high a risk for stroke as patients with high ABCD2 scores but no DWI abnormalities.
Vascular Imaging Studies
Vascular imaging for TIA includes Doppler ultrasonography, CT angiography (CTA), and magnetic resonance angiography (MRA). CTA is of increasing value in identifying occlusive disease in the cerebrovascular circulation. MRA is another alternative for imaging vessels in both the brain and the neck. Conventional catheter angiography can be performed when the other modalities are unavailable or yield discordant results.
Carotid Doppler ultrasonography of the neck can be used to identify patients in need of urgent surgical or endovascular therapy. Transcranial Doppler can be a complementary examination evaluating the patency of cerebral vessels and collateral circulation.
Electroencephalography & Lumbar Puncture
Electroencephalography (EEG) may be indicated to evaluate for seizure activity. Lumbar puncture (LP) may be indicated if subarachnoid hemorrhage, central nervous system (CNS) infection, or demyelinating disease is to be excluded
Laboratory Studies
The following tests are considered on an emergency basis:
- Serum chemistry profile, including creatinine
- Screening coagulation studies
- CBC
The following tests may be helpful and often can be performed on an urgent basis:
- Erythrocyte sedimentation rate (ESR)
- Cardiac enzymes
- Lipid profile
Screening for hypercoagulable states (particularly in younger patients with no known vascular risk factors) may be performed, though this practice is not evidence-based. Tests include the following:
- Protein C, protein S, and antithrombin III activities
- Activated protein C resistance/factor V Leiden
- Fibrinogen
- D-dimer
- Anticardiolipin antibody
- Lupus anticoagulant
- Homocysteine
- Prothrombin gene G20210A mutation
- Factor VIII
- Von Willebrand factor
- Plasminogen activator inhibitor-1
- Endogenous tissue plasminogen activator activity
Additional laboratory tests, ordered as needed and on the basis of the history and examination, include the following:
- Syphilis serology
- Antiphospholipid antibodies
- Toxicology screens
- Hemoglobin electrophoresis
- Serum protein electrophoresis
- Cerebrospinal fluid examination
Differential Diagnoses
- Carotid Artery Dissection
- Stroke, Hemorrhagic
- Stroke, Ischemic
- Subarachnoid Hemorrhage
- Tumor or mass lesion
- Meningitis
- Syncope
- Migraine with aura
- Peripheral nerve/root disorder
- Demyelinating disease
- Vestibular dysfunction
- Electrolyte derangements
- Drug-induced conditions
- Metabolic conditions
Diagnostic Considerations
It has been suggested that transient ischemic attacks (TIAs) are a subset of a larger category termed transient neurologic attacks (TNAs). TNAs are defined as episodes of sudden onset of neurological symptoms that completely resolve within 24 hours and have no clear diagnosis. When a TNA is associated with focal symptoms attributed to an arterial territory of the brain, it is consistent with a stroke or TIA and is termed a focal TNA.
In contrast, a nonfocal TNA is a temporary event of diffuse, nonlocalizing, cerebral symptoms that set in suddenly and resolve quickly. Symptoms of nonfocal TNAs may include the following:
- Altered consciousness
- Nonrotatory dizziness
- Positive visual phenomena
- Paresthesias
- Bilateral weakness
- Generalized feelings of unwellness with a clinical suspicion of neurologic disease
When symptoms are both focal and nonfocal, the term “mixed TNA” would apply.
Nonfocal TNAs have long been considered benign. One study has shown, however, that patients who had suffered a prior nonfocal TNA were at higher risk of stroke and dementia, especially vascular dementia, than patients who did not have a history of prior TNA.
Although more research is required, this study challenges the idea that nonfocal TNAs are benign events. Further workup should be considered in patients suffering nonfocal TNAs to elucidate the underlying cause of their transient symptoms and to achieve better risk stratification.
Patient Disposition
Although controversy exists regarding the need for hospital admission, there is no controversy regarding the need for urgent evaluation, risk stratification, and initiation of stroke prevention therapy. When one community implemented a strategy to ensure patients were seen within an average of 1 day, compared with an average of 3 days, the 90-day stroke risk fell from 10% to 2%.
Similarly, programs to admit patients to a “rapid evaluation unit” or “observation unit” have reduced the 90-day stroke risk from approximately 10% to 4-5%.
The availability of local resources determines whether this urgent evaluation should occur on an inpatient basis, in an ED observation unit, or in rapid follow-up. To determine appropriate disposition, the emergency physician should decide on the necessary workup, then discuss with the neurologist or primary care physician how best to ensure that this occurs promptly.
One randomized controlled trial of an ED diagnostic protocol found that it was possible to reduce cost, shorten length of stay, and provide appropriate risk stratification by performing this workup in an ED observation unit (with neurology consultation) rather than in an inpatient unit. On the other hand, admission offers the potential benefit of decreased time to thrombolysis in hospitalized patients diagnosed with TIA who develop a new ischemic stroke in the first 24-48 hours after diagnosis.
The American Heart Association (AHA) suggests hospital admission as a reasonable choice for patients with TIA if they present within 72 hours of the event and meet any of the following criteria:
- ABCD 2 score of 3 (class IIa recommendation; evidence level C)
- ABCD 2 score of 0-2 and uncertainty that diagnostic workup can be completed within 2 days as an outpatient (class IIa recommendation; evidence level C)
- ABCD 2 score of 0-2 and other evidence that indicates the patient’s event was caused by focal ischemia (class IIa recommendation; evidence level C)
The National Stroke Association consensus guidelines for the management of TIAs recommend considering patient hospitalization if it is the first TIA within the previous 24-48 hours. This would facilitate possible early treatment with tissue plasminogen activator (tPA) and other medical management for recurrent symptoms, and it would expedite risk stratification and implementation of secondary prevention.
For patients with a recent (≤1 week) TIA, the guidelines recommend a timely hospital referral with hospitalization for the following:
- Crescendo TIAs
- Symptoms lasting longer than 1 hour
- Symptomatic internal carotid stenosis greater than 50%
- Known cardiac source of embolus (e.g., atrial fibrillation)
- Known hypercoagulable state
- Appropriate combination of the California score or ABCD score
Treatment & Management
Management of Hypertension
Patients may be significantly hypertensive. Unless there is specific concern for end-organ damage from a hypertensive emergency, blood pressure should be managed conservatively while ischemic stroke is being ruled out.
For acute ischemic stroke, the AHA recommends initiating antihypertensive therapy only if blood pressure is higher than 220/120 mm Hg or if mean arterial pressure exceeds 130 mm Hg. Unless there is a comorbid cardiac or other condition that necessitates reduction of blood pressure, allowing the patient’s blood pressure to autoregulate at a higher level (during the acute phase) may help maximize cerebral perfusion pressure.
Pharmacologic Therapy
In view of the high short-term risk of stroke after TIA, antithrombotic therapy should be initiated as soon as intracranial hemorrhage has been ruled out. The guidelines developed by the AHA and the American Stroke Association (ASA) for the prevention of stroke in patients with stroke or TIA, issued in 2006 and updated in 2014, are summarized below.
Non-Cardioembolic Transient Ischemic Attack
Antiplatelet agents, rather than oral anticoagulants, are recommended as initial therapy. Aspirin 50-325 mg/day, a combination of aspirin and extended-release dipyridamole, and clopidogrel are all reasonable first-line options (class I recommendation).
The AHA/ASA guidelines state that the combination of aspirin and clopidogrel might be considered for initiation within 24 hours of a minor ischemic stroke or TIA and for continuation for 21 days. However, the combination of aspirin and clopidogrel, when initiated days to years after a minor stroke or TIA and continued 2 to 3 years, increases the risk of hemorrhage relative to either agent alone and is not recommended for routine long-term secondary prevention after ischemic stroke or TIA.
Cardioembolic Transient Ischemic Attack
In patients who have atrial fibrillation in association with a Transient Ischemic Attack (TIA), long-term anticoagulation with warfarin to a target international normalized ratio (INR) of 2-3 is typically recommended. Aspirin 325 mg/day is recommended for patients unable to take oral anticoagulants. However, the addition of clopidogrel to aspirin therapy, compared with aspirin therapy alone, might be reasonable. For most patients with a stroke or TIA in the setting of AF, it is reasonable to initiate oral anticoagulation within 14 days after the onset of neurological symptoms. Anticoagulation can be delayed beyond 14 days in the presence of high risk for hemorrhagic conversion.
The 2014 AHA/ASA guidelines also state that bridging therapy with subcutaneous low-molecular-weight heparin (LMWH) is reasonable for patients with atrial fibrillation who require temporary interruption of oral anticoagulation but are at high risk for stroke.
In acute myocardial infarction (MI) with left ventricular thrombus, oral anticoagulation with warfarin (target INR, 2-3) is reasonable.
In dilated cardiomyopathy, either oral anticoagulation with warfarin (target INR, 2-3) or antiplatelet therapy may be considered. In rheumatic mitral valve disease, oral anticoagulation with warfarin (target INR, 2-3) is reasonable. Antiplatelet agents would not normally be added to warfarin unless patients experience recurrent embolism despite a therapeutic INR. The benefit of warfarin after stroke or TIA in patients with sinus rhythm and cardiomyopathy characterized by systolic dysfunction has not been established.
In mitral valve prolapse, long-term antiplatelet therapy is reasonable. In mitral annular calcification, antiplatelet therapy can be considered. Patients with mitral regurgitation can be considered for warfarin or antiplatelet therapy.
In aortic valve disease, antiplatelet therapy may be considered. For patients with mechanical prosthetic valves, oral anticoagulation with warfarin (target INR, 2.5-3.5) is recommended. For those who experience TIAs despite therapeutic INR, aspirin 75-100 mg/day can be added to the regimen. Patients with bioprosthetic valves and no other source of thromboembolism who experience TIAs can be considered for oral anticoagulation with warfarin (target INR, 2-3).
Large-Artery Atherosclerotic Disease
Intracranial atherosclerosis
The 2014 AHA/ASA guidelines state the following for patients with stroke or TIA due to 50-99% stenosis of a major intracranial artery:
- Aspirin 50-325 mg/day, rather than warfarin, is recommended
- Maintenance of blood pressure below 140/90 mm Hg and total cholesterol below 200 mg/dL is recommended
- Extracranial or intracranial bypass surgery is not recommended
- Angioplasty and stent placement are investigational and of unknown utility
A randomized trial has shown that aggressive medical management (antiplatelet therapy combined with intensive management of vascular risk factors) is safer than percutaneous transluminal angioplasty and stenting (PTAS) in patient with 70-99% stenosis of a major intracranial artery. Enrollment in this trial was stopped after 451 patients underwent randomization because the 30-day rate of stroke or death was 14.7% in the PTAS group and 5.8% in the medical-management group.
Ipsilateral Carotid Artery Stenosis
Patients with TIA and ipsilateral carotid artery stenosis may be candidates for urgent (performed within 2 weeks) carotid endarterectomy. In certain patients, carotid artery angioplasty and stenting is a reasonable alternative. This can be discussed acutely, or rapid follow-up can be arranged.
Extracranial Vertebral Stenosis
Patients with symptoms attributable to extracranial vertebral stenosis may be candidates for endovascular treatment. Again, this should be arranged expeditiously if available.
According to the AHA/ASA 2014 guidelines, optimal medical treatment for these patients includes antiplatelet and statin therapies, as well as risk factor modification. This is also optimal medical treatment for patients with symptomatic extracranial carotid disease.
Consultations
Ideally, decisions regarding ED evaluation and inpatient versus rapid outpatient follow-up are made in concert with a neurologist. There is clear consensus on the importance of rapid evaluation. For example, in the EXPRESS (Early use of eXisting PREventive Strategies for Stroke) study, the 90-day risk of recurrent stroke in patients with TIA or minor stroke was 10.3% in those patients who underwent assessment after a median of 3 days, compared with 2.1% in those assessed in a median of 1 day, who then received prompt treatment.
In some settings, the only way to access expedited evaluation and workup may be through interfacility transfer to a hospital with the appropriate resources. The National Stroke Association consensus guidelines recommend that “[h]ospitals and general practitioners should agree on a local admissions policy and a local protocol for referral to specialist assessment clinics for patients with TIA who do not require hospital admission.”
For ED physicians, consultation with the patient’s primary care physician is the most important consultation because the primary care physician will monitor the patient over the long term and ensure risk-factor and lifestyle modification. In addition, a rapid neurology consultation is not available in many communities, and the primary care doctor may be primarily responsible for managing urgent risk stratification. However, when a neurologist is rapidly available, this consultation should be obtained on an urgent basis as well.
Consultation with a cardiologist can be considered for patients with clear cardiac findings that influence stroke risk, such as atrial fibrillation, patent foramen ovale, intracardiac thrombus, or valvular abnormalities.
Consultation with a neurosurgeon or vascular surgeon should be considered for patients with significant vessel stenosis or occlusion, with a goal of specialist assessment within 1 week and treatment within 2 weeks of symptom onset. In many centers, some endovascular interventions can be performed by other specialists, including interventional neurologists, radiologists, and neuroradiologists.
Long-Term Monitoring
Patients selected for outpatient care should have a clear follow-up plan and stroke prevention initiated as described, including antiplatelet medication and risk-factor modification. Antiplatelet agents typically should be initiated as soon as intracranial bleeding is ruled out.
The following measures should be included in any long-term monitoring of TIA patients:
- Antihypertensive control should be optimized for patients with hypertension
- Lipid control should be initiated, potentially including a statin agent
- Blood glucose control should be optimized for patients with diabetes
- A smoking-cessation strategy, which may include medication, should be initiated
- Heavy drinkers should eliminate or reduce alcohol consumption
- Overweight patients should be encouraged to lose weight
- All patients should be encouraged to exercise
Medication Summary
Pharmacologic management for transient ischemic attacks (TIAs) is aimed at reducing both short-term and long-term risk of stroke. In view of the high short-term risk of stroke after TIA, antithrombotic therapy should be initiated as soon as intracranial hemorrhage has been ruled out.
Antiplatelet Agents
Class Summary
Antiplatelet agents inhibit platelet function by blocking cyclooxygenase and subsequent aggregation.
Aspirin (Anacin, Ascriptin, Ecotrin, Bufferin, Bayer Aspirin)
Aspirin blocks prostaglandin synthetase action, and this, in turn, inhibits prostaglandin synthesis and prevents formation of platelet-aggregating thromboxane A2.
Aspirin 25 mg/dipyridamole 200 mg (Aggrenox)
Combination aspirin-dipyridamole therapy has been shown to prevent cardiovascular events following TIAs. Each capsule contains 25 mg of aspirin and 200 mg of dipyridamole, for a daily total dose of 50 mg of aspirin and 400 mg of dipyridamole.
Aspirin irreversibly inhibits formation of cyclooxygenase, thus preventing formation of thromboxane A2, a platelet aggregator and vasoconstrictor. Platelet inhibition lasts for the life of a cell (approximately 10 days).
Dipyridamole is a platelet adhesion inhibitor that possibly inhibits red blood cell (RBC) uptake of adenosine, itself an inhibitor of platelet reactivity. In addition, it may inhibit phosphodiesterase activity, leading to increased cyclic 3′,5′-adenosine monophosphate within platelets and formation of the potent platelet activator thromboxane A2.
Clopidogrel (Plavix)
Clopidogrel selectively inhibits the binding of adenosine diphosphate (ADP) to its platelet receptor and subsequent ADP-mediated activation of the glycoprotein GPIIb/IIIa complex, thereby inhibiting platelet aggregation.
Dipyridamole (Persantine)
Dipyridamole is administered to complement usual warfarin therapy. It inhibits platelet adhesion, which may inhibit adenosine uptake by RBCs. It may increase cyclic 3′,5′-adenosine monophosphate (cAMP) within platelets and formation of the potent platelet activator thromboxane A2. In addition, it may reduce the risk of stroke when used as monotherapy instead of aspirin.
Ticlopidine (Ticlid)
Ticlopidine is a second-line antiplatelet therapy for patients who cannot tolerate or do not respond to aspirin therapy. In some circumstances, it can be an alternative to clopidogrel.
Anticoagulants
Class Summary
Controlled therapeutic inhibition of blood clotting by means of appropriate drugs (i.e., anticoagulants) is indicated for prevention of ischemic stroke in patients with risk factors for thromboembolism, such as atrial fibrillation.
Warfarin (Coumadin)
Warfarin interferes with hepatic synthesis of vitamin K−dependent coagulation factors. It is used for prophylaxis and treatment of venous thrombosis, pulmonary embolism, and thromboembolic disorders.
Prognosis
In people who have a TIA, the early risk of stroke is approximately 4% at 2 days, 8% at 30 days, and 9% at 90 days. When patients with TIA are followed prospectively, however, the incidence of stroke is as high as 11% at 7 days. The probability of stroke in the 5 years following a TIA is reported to be 24-29%. In addition, patients with TIAs or stroke have an increased risk of coronary artery disease.
Patient Education
Before being discharged from the hospital, patients who have been diagnosed with TIA must receive clear instruction to ensure that they understand the need for a complete and rapid workup through close follow-up care. Also essential for patients is education on stroke symptoms, the need to call emergency services immediately if any of these symptoms occur.
In addition, patients need to be educated about lifestyle modification and cardiovascular risk factors.


Leave a Reply
Want to join the discussion?Feel free to contribute!