Posttraumatic Hydrocephalus
Posttraumatic hydrocephalus (PTH) is a frequent and serious complication that follows a traumatic brain injury (TBI). PTH could greatly impact morbidity following a TBI and could result in increased mortality if it is not recognized and treated.
PTH may result from 1 or a combination of pathophysiologic factors. It can be caused by the overproduction of cerebrospinal fluid (CSF), the blockage of normal CSF flow, or insufficient absorption that results in excessive accumulation of CSF around the brain. Ultimately, PTH is caused by an imbalance that occurs between CSF production and absorption.
PTH may present as normal pressure hydrocephalus (NPH) or as a syndrome of increased intracranial pressure. Because of differences in prognosis and treatment, PTH needs to be distinguished from cerebral atrophy (i.e., hydrocephalus ex vacuo) and ventricular enlargement caused by a failure of brain development. If PTH goes unrecognized or untreated, increased morbidity or mortality following a TBI is more likely.
Classification
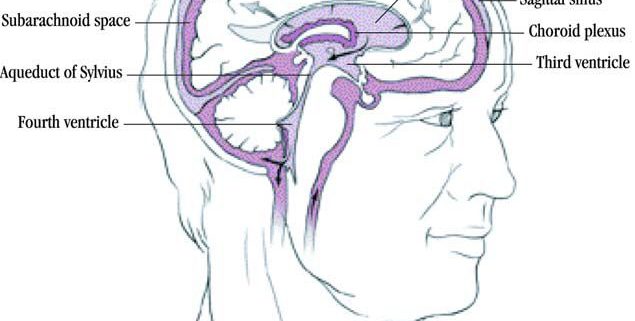
Hydrocephalus is classified as either noncommunicating or communicating.In noncommunicating hydrocephalus (also called obstructive hydrocephalus), CSF accumulates in the ventricles because of CSF flow blockage. As a result, the ventriclesenlarge and the hemispheres expand. The following sites are prone to the obstruction of CSF flow:
- Foramen of Monro
- Third ventricle
- Aqueduct of Sylvius
- Fourth ventricle
- Foramen of Luschka
- Foramen of Magendie
Conversely, in communicating hydrocephalus (also referred to as nonobstructive hydrocephalus), full communication between the ventricles and the subarachnoid space exists. Impaired CSF absorption may cause communicating hydrocephalus. The apparent mechanism is partial occlusion of the arachnoid villi, perhaps by blood and inflammatory mediators. Severe skull fractures, hemorrhage, and meningitis may predispose patients to this variant of PTH. Another possible mechanism is increased duralsinus pressure, causing decreased CSF outflow.
NPH, a form of communicating hydrocephalus, may result from subarachnoid hemorrhage caused by an aneurysm rupture or a TBI, encephalopathy, or Alzheimer disease. NPH often presents as the classic triad of a progressive gait disorder, impairment of mental function, and urinary incontinence. In NPH, ventricles enlarge despite normal or even slightly reduced intracranial pressure, and they continue to press against brain parenchyma.
Causes
Obstructive hydrocephalus may be caused by tumors, infection, abscesses, cysts, or trauma. NPH may result from a subarachnoid hemorrhage caused by an aneurysm rupture, a TBI, or encephalopathy.
Older age and low consciousness level are independent risk factors for the development of PTH during rehabilitation in patients with severe TBI.
A retrospective study by Di et al. reported that other risk factors for the development of PTH in TBI patients who undergo decompressive craniectomy include a Glasgow Coma Scale score of less than 6 on admission, a finding of intraventricular hemorrhage on initial head CT scan, and a requirement for a bilateral decompressive craniectomy.
Another study, by Vedantam et al., indicated that in cases of severe TBI treated with decompressive craniectomy, patients with interhemispheric subdural hygromas and of younger age are at greater risk for the development of shunt-dependent PTH.
Normal anatomy and physiology
- normal intracranial pressure (ICP) is approximately 8 mm Hg.
- The average intracranial volume is about 1700 mL.
- The average CSF volume is about 104 mL.
By volume, the intracranial contents include the following:
- Brain parenchyma – About 80%
- CSF – About 10%
- Blood – About 10%
CSF is primarily produced in the lateral ventricles by the choroids plexus at a rate of 500 mL/d. The CSF flows down toward the third ventricle through the foramen of Monro and into the fourth ventricle through the cerebral aqueducts. The CSF then exits the ventricular system through the foramen of Magendie (medially) and the foramen of Luschka (literally) and flows into the perimedullary and perispinal subarachnoid spaces. The CSF continues around the brainstem to the basal and ambient cisterns. It then flows to the lateral and superior surfaces of the cerebral hemispheres, where it is largely absorbed through the arachnoid villi. The total volume of CSF is replaced several times daily.
Frequency
The onset of PTH may vary from 2 weeks to years after TBI. Studies cite a wide range of incidence (0.7-50%); part of this variation results from underdiagnosis and atypical presentation, as well as from the fact that different sets of clinical criteria are used to diagnose PTH.
Mazzini and colleagues found that 50% of patients with post-acute phase severe TBI had PTH but that only 11% required surgery.
In a multi-year study, Kim and colleagues followed 789 patients who had suffered a TBI, diagnosing PTH in 129 (16.3%) of them. Sixty-four patients with PTH required shunting.
History
Posttraumatic hydrocephalus (PTH) often has an atypical presentation and therefore may be easily missed. A high level of clinical suspicion is important for diagnosis.
- If acute, patients may present with coma and other focal neurologic deficits.
- If chronic, patients may demonstrate a gradual decline in functional status or may show a failure to improve. The decline in performance or functioning may be initially observed by therapists.
The cognitive decline of NPH may present similarly to dementias in elderly patients. However, Alzheimer disease more commonly presents insidiously over a period of years, with progressive memory impairment, anomia, apraxia, agnosia, and a decline in executive function. Dementia in NPH usually presents with subcortical features.
Physical
See the list below:
- A complete neurological examination should be performed and repeated as necessary to assess for changes over time.
- PTH may present as a syndrome of increased intracranial pressure with symptoms of papilledema, focal neurologic deficits, or coma.
- Most commonly, PTH presents as noncommunicating hydrocephalus with the following findings:
- Papilledema resulting from increased intracranial pressure and transmission through the subarachnoid space
- Cognitive changes, including decreased memory, decreased attention, and irritability
- NPH often presents as a classic triad of the following symptoms:
- Progressive gait disorder
- Impairment of mental function
- Urinary incontinence
- In NPH, headaches are not common, and papilledema is not usually seen on funduscopic examination; patients may present with frontal release signs.
Diagnostic Considerations
These include the following:
- Intracranial bleeding
- Electrolyte imbalance
- Adverse effects of medications
- Hypoxia
- Infection
- Tumors
- Stroke
- Seizures
- Uremia
- Encephalopathy
- Dementia
Workup
Pertinent laboratory studies include the following:
- Urine analysis and culture – Evaluate for urinary tract infections
- Complete blood count (CBC) with differential – Evaluate for infection and anemia
- Metabolic profile – Evaluate for electrolyte abnormalities
- Thyroid-stimulating hormone (TSH), free thyroxine (free T4) – Evaluate for hypothyroidism or hyperthyroidism
- Arterial blood gas level – Assess oxygenation and acid/base balance
- Serum medication levels – Measure medication levels if toxicity suspected
Imaging Studies
See the list below:
- Noncontrast CT scan of the brain is one of the most commonly used diagnostic modalities.
- The progressive enlargement of the ventricular system shown on repeat computed tomography (CT) scans is the key to the diagnosis of PTH.
- CT scans may show enlarged lateral ventricles, effaced cerebral sulci, and dilation on ventricles proximal to an obstruction.
- Periventricular edema may occur in white matter, particularly around the frontal horns.
- Sulcal enlargement with ventricular enlargement suggests atrophy and hydrocephalus ex vacuo rather than hydrocephalus.
- Large cisterns and focal regions of encephalomalacia suggest atrophy.
- Magnetic resonance imaging (MRI) is another method of diagnostic evaluation.
- MRI is more useful in the evaluation of injury to structures in the posterior fossa, including cerebral aqueduct stenosis and cerebellar tonsil herniation.
- It is the neuroimaging study of choice in patients with NPH.
- MRI may be more useful than CT scanning in the identification of other neurologic disorders, especially cerebrovascular disease.
Mazzini studied another imaging technique, single-photon emission CT (SPECT). Mazzini found that SPECT had higher sensitivity than MRI or CT scanning in the demonstration of temporal lobe abnormality secondary to PTH.
Other Tests
See the list below:
- Radionuclide cisternography:
- Radioiodinated serum albumin (RISA) injected into the subarachnoid space by way of lumbar puncture (LP) can normally be detected in the cisterna magna, basal cisterns, and subtentorial subarachnoid space within 6 hours, with little accumulation in the ventricular system. In NPH, RISA accumulates in the ventricular system with delayed pericerebral diffusion.
- Cisternography is usually normal in hydrocephalus ex vacuo.
- Although debate exists, cisternography may be a useful adjunct to CT scanning of the brain.
Procedures
See the list below:
- CSF tap test
- This test is an LP with manometry and CSF removal.
- Imaging of the brain should be performed before initiating the LP. The risk of cerebral herniation associated with the LP is increased in patients with greatly elevated ICP.
- The CSF tap test may be a useful predictor of the potential benefits of shunting. Kim (2005) found that symptomatic improvement after lumbar drainage has a significant role in predicting the result of shunting.
- CSF pressure is normally 110 mm water. Shunting may help if the pressure is 135-275 mm water, and it does help if the pressure is greater than 275 mm water.
- Cognitive and physical functions are assessed before and after the removal of 50 mL of CSF. Improvement suggests that shunting may be beneficial.
Management
Shunting is the most common treatment for hydrocephalus. The outcome is typically favorable. A shunt is usually placed from the right ventricle to the peritoneal space. The right side is normally used to avoid injury to the language centers on the left side of the brain. Shunts are most often equipped with reservoirs that are used for transiently increasing output and for testing the patency of flow.
The resumption of rehabilitation is usually prompt after the placement of a ventriculoperitoneal (VP) shunt. Patients are typically observed for 2-3 days postoperatively. They then return to rehabilitation services to complete their brain-injury rehabilitation program. Successful shunting is usually related to more obvious and rapid improvements during rehabilitation efforts.
Rehabilitation Program
Physical Therapy
The resumption of rehabilitation is usually prompt after the placement of a ventriculoperitoneal (VP) shunt. Patients are typically observed for 2-3 days postoperatively. They then return to rehabilitation services to complete their brain-injury rehabilitation program. Successful shunting is usually related to more obvious and rapid improvements during rehabilitation efforts.
A study by Weintraub et al indicated that earlier shunting in posttraumatic hydrocephalus (PTH) is associated with improved rehabilitation outcomes. The study involved 52 PTH patients who had a VP shunt placed, with the period from injury to placement ranging from 9 to 366 days (median time, 69 days).
- Occupational Therapy
- Speech Therapy
- Recreational Therapy
Mortality/Morbidity
If PTH goes unrecognized or untreated, increased morbidity or mortality following a TBI is more likely.


Leave a Reply
Want to join the discussion?Feel free to contribute!