Common Errors Made in the Diagnosis and Treatment of Epilepsy
Abstract
This article is a compilation of some of the frequent mistakes that are made in the evaluation and management of patients with epilepsy. It encompasses errors in the clinical diagnosis that result in the choice of the erroneous antiepileptic drug (AED), errors in the way auxiliary tests like the electroencephalogram and magnetic resonance imaging studies are ordered, mistakes in the recognition of subclinical status epilepticus, errors in the selection of AEDs, consequences of the failure to factor in the pharmacokinetic and pharmacodynamic properties of AEDs in the choice and dosage of medication, misconceptions on the expectations of therapeutic effect of AEDs, delay in recognition of refractory epilepsy with consequent delay in a timely identification of patients whose epilepsy can be cured with surgical treatment, and mistakes in the recognition and management of comorbid psychiatric disorders. In addition to a discussion of the reasons for the errors, the article provides practical solutions.
Introduction
The purpose of this article is to review some of the most frequent mistakes in the evaluation and management of children and adults with epilepsy. It focuses primarily on those mistakes that prevent seizure freedom, those that result in a worsening of patients’ quality of life, and those with the greatest potential to improve morbidity and mortality rates.
The article is divided into three sections. The first is a discussion of common mistakes made in the diagnosis of epileptic seizure disorders, both with respect to reaching a clinical diagnosis of an epileptic syndrome and the implications that this may have on its management. The second section addresses common mistakes made in the management of epileptic disorders, devoting the first part to pharmacotherapy and the second to surgical treatment. Finally, the third section reviews the impact of the failure to recognize and treat comorbid cognitive and psychiatric conditions that are common in epilepsy and offers very practical approaches to the controversial use of psychotropic drugs in these patients.
Common Errors in Diagnosis
Failure to get a good and detailed history is the most frequent cause of diagnostic errors in any of the medical fields, and epilepsy is not an exception. In fact, in patients with epilepsy, a detailed history is likely to lead to an accurate diagnosis in up to 90% of patients. In such cases, auxiliary studies help to confirm the diagnosis. In the evaluation of patients with a possible diagnosis of epilepsy, the first task is to establish whether the paroxysmal episode under investigation is, in fact, epileptic or nonepileptic. If the clinical features of the event are suggestive of an epileptic seizure, the next step is to establish the type of seizure and epileptic syndrome, and whether the seizure in question was the first epileptic seizure ever, including seizures of other types that have gone unrecognized by the patient or family.
If the event is suspected to be nonepileptic, it is necessary to establish if it may be organic (i.e., syncope, sleep disorder, movement disorder, etc.) or psychogenic. The misdiagnosis of nonepileptic events as epileptic seizures is a relatively frequent occurrence. Indeed, 1 of 4 to 5 patients admitted to a video-electroencephalogram (EEG) monitoring unit with a diagnosis of pharmacoresistant epilepsy is found to have nonepileptic events, the majority of which are of psychogenic origin. In recent years, however, as clinicians are becoming more aware of psychogenic nonepileptic events, epileptic seizures with bizarre clinical characteristics are being misdiagnosed as the former. Such is the case of frontal lobe seizures.
Case 1. A 20-year-old woman was brought to the emergency room after having been witnessed to have an unprovoked generalized tonic-clonic seizure (GTC). She underwent computerized tomography (CT) scan of the brain that was normal; laboratory evaluation included a hemogram (complete blood count), a chemistry panel, and a toxicology screen, all of which were unremarkable. She was discharged home and was told that she did not require any medication since this was “a first unprovoked seizure.” Five months later she was brought back to the emergency room after she experienced a second GTC seizure. She reported having been sleep-deprived the previous 2-days and having had five alcoholic drinks the night before. During the exam, she was noticed to exhibit episodes of motionless staring of up to 15 seconds’ duration during which she appeared to be unresponsive. She was not aware of these episodes but stated that occasionally people would tell her that they would be talking to her and she appeared to be “distracted.” Additional history obtained revealed the presence of a whole body or upper extremity jerks for the previous 9-months that tended to occur early after waking up, especially if she had been under stress or sleep deprived. She had always attributed these jerks to stress. An EEG revealed a pattern of polyspikes and slow waves with a generalized distribution, consistent with a diagnosis of juvenile myoclonic epilepsy (JME).
Where was the mistake? This case illustrates a common assumption that a first GTC seizure must be the patient’s first epileptic seizure. This error, which would have been averted had a careful investigation of previous seizures been undertaken in the emergency room, resulted from an “incomplete” history. It is not enough to rely only on generic questions, such as “have you had any seizures in the past?” without providing patients with a description of the various seizure types that often go unrecognized. These include myoclonic and simple partial seizures presenting as epigastric discomfort, sensations of déjà vu, jamais vu, feelings of derealization, brief episodes of vertigo, or shapeless visual hallucinations lateralized to one hemifield. Additionally, there are seizures with very brief loss of awareness that patients are often unaware of unless these are brought to their attention by witnesses, such as absence and complex partial seizures of very short duration. In fact, it is not infrequently that patients who are brought to an emergency room with a first unprovoked GTC seizure have been experiencing epileptic seizures for several months, if not years. Such is the case in patients with JME as illustrated above who have been experiencing myoclonic seizures of mild severity and attributing them to a variety of causes, such as nervousness or lack of sleep (as it is not infrequently that clusters of myoclonic seizures may be present following sleep deprivation or alcohol intake). This type of mistake delays the decision to start treatment with an antiepileptic drug (AED). In fact, failure to start treatment after a first GTC seizure in JME is associated with a very high risk of recurrent GTC and myoclonic seizures with the risk of self-harm.
The same observations apply to a first GTC seizure that occurs as part of a partial seizure disorder. It is not unusual for patients to experience recurrent simple partial seizures presenting as “auras.” Frequently, patients who experience epigastric discomfort, a sensation of déjà vu, jamais vu, or panicky feelings ascribe these symptoms to anxiety and are often treated by their family physicians with anxiolytics, when in reality, they have been experiencing simple partial seizures of mesial temporal lobe origin.
Clearly, careful histories must be performed in patients with a first unprovoked GTC seizure to eliminate the possibility of other types of seizures. Identification of a family history of epilepsy can often serve as a red flag that may alert the clinician to the possibility of primary generalized epilepsy.
Case 2. A 7-year-old boy was referred for an evaluation because of sudden deterioration of his academic performance in the previous 3- months. The teacher reported to his parents having witnessed recurrent episodes during which he was staring in a motionless manner for periods of 5 to 20 seconds, the longer ones associated with oral and hand automatisms. There was no postictal confusion. He was diagnosed with a partial seizure disorder and started on carbamazepine (CBZ). Four weeks later, the seizures had worsened in frequency and duration to the point where he appeared confused. An EEG revealed very frequent runs of 3-Hz spike-wave complexes with a generalized distribution and recurrent electrographic and clinical seizures of 5 to 30 seconds’ duration. His treatment was changed to ethosuximide, resulting in the complete remission of his seizures.
This case illustrates another frequent mistake consisting of a failure to recognize the correct type of epileptic seizure and syndrome before the start of therapy. The history was highly suggestive of an absence seizure disorder. When partial seizures of frontal lobe origin present in this manner, an EEG can clarify the diagnosis. The erroneous diagnosis can result in the wrong selection of AED that can worsen the seizure frequency, as illustrated by this case. In addition to worsening absence seizures, CBZ and phenytoin can worsen myoclonic seizures in patients with primary generalized epilepsy.
Thus, before any AED prescription is written, clinicians must ask themselves two questions: (1) Have I obtained all the necessary data to identify the type of epileptic seizure and the syndrome of this patient? (2) Has this AED been shown to be effective for the type of epileptic seizure and the epileptic syndrome that I suspect the patient has? The use of AEDs with “broad-spectrum” efficacy may simplify the choice of AED, but it is not an excuse for not having established a correct diagnosis, as it also provides an expectation of a response to pharmacotherapy. Indeed, response to pharmacotherapy differs significantly among the various epileptic syndromes. For example, patients with a partial seizure disorder of mesial frontal origin have a 50% probability of becoming seizure-free with pharmacotherapy, while patients with childhood absences have up to an 80% probability of becoming seizure-free on the right antiepileptic medications.
Case 3. A 58-year-old woman underwent the evacuation of a left intraparenchymal frontal abscess. The night after the surgical procedure, she was witnessed to experience four GTC seizures, which were treated with 6 mg of lorazepam intravenous (IV) and 1500 mg of IV fosphenytoin. While no further GTC seizures recurred, she remained unresponsive (including to noxious stimulation) after the administration of IV AEDs, which was attributed to the sedative effect of lorazepam. The following morning, she remained unresponsive. On exam, she displayed nystagmoid movements of the eyes to the right and subtle irregular clonic jerks of the right toes. An EEG recording revealed the presence of ictal activity.
This case illustrates the mistaken assumption that the absence of any “overt” clinical epileptic activity implies remission of seizure activity in patients who have experienced a flurry of epileptic seizures (and/or clear-cut status epilepticus). Unless the patient becomes responsive, remission of seizure activity should not be assumed unless confirmed with EEG recordings.
Approximately 60% of patients with convulsive status epilepticus are expected to respond to first-line pharmacotherapy. When the first drug fails to control status epilepticus, the addition of a second drug increases the remission of seizure activity by an additional 7 to 10%. Hence, 30% of patients with status epilepticus are expected to continue having epileptic activity, often presenting as nonconvulsive status epilepticus or subtle GCSE. This patient illustrates such a case.
The diagnosis of subtle GCSE is often missed by clinicians, as it presents with subtle signs, such as the eyelid, facial, or jaw twitching, rhythmic nystagmoid eye jerks, or subtle rhythmic focal twitching of trunk or extremities. The diagnosis is made by EEG recordings. It is not infrequently that before subtle GCSE becomes apparent, patients will have had several overt GTC seizures, and clinicians assume that the initial antiepileptic medication resulted in seizure remission because of the absence of any obvious evidence of clonic activity of the extremities. These patients, however, do not regain consciousness and failure to regain consciousness is often misinterpreted by Clinicians as being secondary to the use of parenteral benzodiazepines or phenobarbital.
The importance of early recognition of status epilepticus has direct implications to the success of therapy.
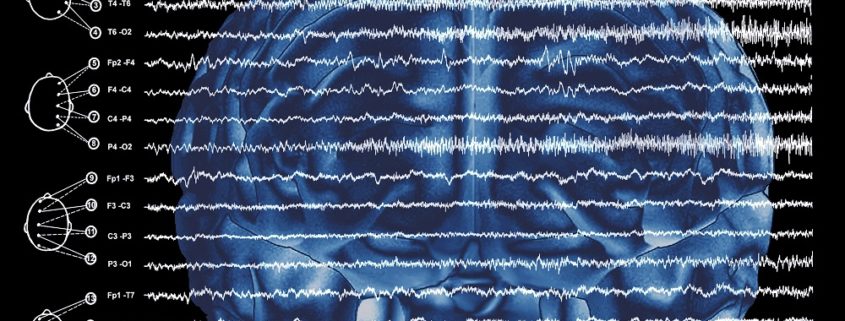
EEG recordings are essential to guide the pharmacologic treatment of status epilepticus, especially when overt status epilepticus has evolved into a subtle form, as this condition can only be objectively evaluated with electrographic recordings. Indeed, without EEG recordings, clinicians have no way of knowing whether the target doses of parenteral antiepileptic medications (or anesthetic agents) have been successful in achieving remission of epileptic activity. This is even more important when inducing coma (i.e., with midazolam, propofol, pentobarbital) in patients with refractory status epilepticus. In coma protocols, the EEG recordings are the only tool to demonstrate the abolition of epileptiform activity and/or the target EEG pattern, which will be maintained for a period of 12 to 24 hours (i.e., burst-suppression pattern or generalized slow-wave activity without epileptiform activity). Common mistakes include failure to use EEG recordings when starting a coma protocol or the use of short EEG studies in the intensive care unit on a daily basis without more prolonged EEG monitoring of the electrical activity, without which it is impossible for clinicians to identify recurrence of epileptic activity.
Case 4. A 25-year-old woman was taken to the emergency room after having had an episode in which she lost consciousness for 30 to 40 seconds while talking on the telephone with a client. She stated that she recalled becoming distressed by the client who was complaining to her about an error she had made in his order. She was not confused after regaining consciousness but had a severe headache. In the emergency room, she underwent a brain CT and a lumbar puncture, both of which were normal, and she was admitted for 24 hours’ observation. An EEG done the following morning was reported to show epileptiform activity in the left temporal region. Accordingly, she was advised to start on AED.
The EEG was misinterpreted. It did not show an electrographic pattern suggestive of epileptiform discharges. It showed a benign variant known as “rhythm enarceau” or wicket spikes. Other benign variants that are often confused with epileptiform discharges are: psychomotor variant, also known as rhythmic midtemporal theta of drowsiness; small sharp spikes of sleep; and subclinical rhythmic electrographic (theta) discharge in adults which consists of periodic sharp activity that evolves into a rhythmic theta pattern, most often parietal in localization and which is identified in older patients during the awake state.
Case 5. A 54-year-old man who had undergone resection of a subdural hematoma was started on phenytoin by the neurosurgeon to “prevent” the occurrence of seizures. After 2-years on this AED, he requested to have the AED discontinued, as he never experienced a seizure. His physician ordered an EEG prior to its discontinuation. The study was reported to show epileptiform activity in the area of the evacuation. Accordingly, the patient was advised to remain on the AED indefinitely.
A careful review of this recording reveals a breach rhythm, an electrographic pattern associated with a prior craniotomy which yields somewhat sharply contoured morphology and higher voltage of the underlying electrical activity, which in turn is often misread as an epileptiform activity. Another common error illustrated by this case is the prescription of AED on EEG data only, in the absence of epileptic seizures. Indeed, this patient had never experienced an epileptic seizure and there was no reason for him to be on the AED beyond the first postsurgical month, as it has been well established that AEDs do not prevent the development of epileptic seizures.
Other common errors in the use of EEG include:
- Failure to insist on sleep recordings in patients suspected of having epilepsy. Indeed, epileptiform activity is activated during sleep in patients with epilepsy. Thus, without a recording that includes sleep, the study must be considered incomplete.
- Failure to request the use of anterotemporal and/or basal temporal electrodes in routine EEG studies ordered in patients suspected of having seizures of anterotemporal origin. These electrodes increase significantly the localizing yield of epileptiform activity originating in mesial temporal structures that often go undetected with the standard 10 to 20 electrodes.
- The erroneous assumption of psychogenic nonepileptic seizures in patients whose ictal recordings fail to display epileptiform activity. In fact, the electrographic pattern of simple partial seizures can be detected in only 25% of cases with scalp ictal recordings, as the synchronous cortical activation of a 10-cm2 area is necessary for scalp electrodes to record any electrographic pattern. By the same token, interictal or even ictal activity of mesial or orbitofrontal origin or with a source in the amygdala may not be detected on scalp recordings, unless they propagate and involve opercular or temporal lateral neocortex, respectively.
Case 6. A 42-year-old man was referred to the psychiatrist with a diagnosis of psychogenic nonepileptic seizures. He had experienced these events for the past 12 years, initially with a frequency of one to two per month. In the last 3 years, their frequency increased to daily events. Clinically, these seizures consist of recurrent episodes of bizarre and violent movements of the entire body, associated with a monotonous nonverbal vocalization that ends with a tonic contraction in the abduction of the upper extremities. Their duration ranges between 20 and 30 seconds, they occur in clusters, and they have been reported to occur in awake and sleep states. If standing, the patient can fall and get hurt. In the past 3 years, this patient had started complaining of excessive daytime somnolence which interfered with his ability to focus at work, and ultimately this led to his losing his job. He has undergone three video-EEG monitoring studies with scalp recordings, all of which failed to reveal any interictal epileptiform activity or concurrent ictal pattern with the events. On the basis of these negative studies, he was told that his events were not epileptic and that his excessive daytime somnolence was an expression of underlying depression and stress for which he was referred for psychiatric treatment. The patient did not agree with the recommendation and requested a fourth opinion.
This case illustrates a very common mistake based on the assumption that the absence of epileptiform activity on interictal or ictal scalp recordings is indicative of nonepileptic events. The exceptions to this rule include (1) epileptic seizures of mesial frontal or mesial parietal origin, (2) seizures originating in the amygdala, and (3) simple partial seizures. For an epileptiform activity to be detected with scalp recordings, asynchronous activation of 10 cm2 is necessary. The following clinical data should have alerted the clinician to the diagnosis of epileptic seizures: their occurrence during sleep and the tonic posturing in the abduction of the upper extremities which is a specific sign of epileptic involvement of the supplementary sensory-motor area.
The second mistake illustrated in this case is the misinterpretation of excessive daytime somnolence as a symptom of depression or stress. In fact, this symptom is very frequent in patients with frontal lobe epilepsy who experience multiple seizures at night, which in turn results in a significant disruption of their sleep, particularly slow-wave sleep, which results in nonrestorative sleep. In other words, the excessive daytime somnolence was the consequence of the disruption of sleep by clusters of nocturnal seizures.
Finally, this case illustrates a common tendency of clinicians to suggest a psychogenic etiology of a variety of neurologic signs and symptoms in the absence of concrete evidence of psychopathology. Furthermore, it is not unusual for patients to develop “reactive” symptoms of depression and frustration in the setting of chronic neurologic disorders that have failed to be properly diagnosed. In such cases, the psychiatric symptoms are not the cause of the paroxysmal episodes but reflect the consequences and frustrations of unsuccessful evaluations.
Case 7. A 26-year-old man was referred for consultation because of persistent seizures of 16-years’ duration, which failed to remit despite multiple AED trials on mono- and polytherapy. His seizures consisted of an aura described as a sensation of déjà vu followed by epigastric discomfort and then a loss of awareness of his surroundings associated with oral automatisms and postictal dysphasia of 10 minutes’ duration. Two magnetic resonance imaging (MRI) studies had been reported to be “normal.” Accordingly, his neurologist refrained from referring him for presurgical evaluation for fear that he was at high risk of suffering postictal memory deficits. A review of the MRI protocol revealed that the coronal cuts were obtained in an angle oblique to the long axis of the hippocampus. A repeat MRI study with a high-resolution protocol in which gapless coronal cuts were done along the temporal lobe and perpendicular to the axis of the hippocampus revealed left hippocampal atrophy consistent with a diagnosis of mesial temporal sclerosis (MTS).
This case illustrates one of the more frequent mistakes of MRI studies in patients suspected of temporal lobe epilepsy (TLE). The proper diagnosis could have been reached had the protocol described above been followed. The timely recognition of MTS is of the essence as it suggests the unlikely remission of seizures with pharmacotherapy in the case of persistent seizures after two AED trials and the high probability of cure with epilepsy surgery (see below). Clearly, it is not enough to request an MRI study and assume that the neuroradiologist will provide the appropriate protocol for the type of seizure disorder.
Common Errors in the Treatment of Epilepsy
Common Errors in the Pharmacologic Treatment of Epilepsy
Case 8. A 27-year-old man was brought to the emergency room after experiencing a second unprovoked GTC seizure. He had had a first seizure 6 months before. He denied having experienced any other type of seizures. A brain MRI was normal and an EEG with awake and sleep recordings were also unremarkable; he was treated with intravenous phenytoin at a dose of 18 mg/kg and was instructed to start at a daily dose of 300 mg. The day after he was started on this drug, he noticed that he would easily become tired and 2-weeks later he developed a generalized macular-papular rash.
This case shows the very common practice in emergency rooms of an “automatic” parenteral load with phenytoin after an isolated GTC seizure as if failure to discharge patients with high serum concentrations would put them at risk of a recurrent seizure. Undoubtedly, there are patients with new-onset epilepsy with such risk; these include patients with an acute neurologic insult such as a stroke with marked edema, a brain tumor with edema, or acute infectious processes such as encephalitis, meningitis, or a brain abscess. It would not be surprising if a significant number of clinicians took issue with the characterization of this practice as an error. Yet, the start of therapy in patients with newly diagnosed epilepsy must be tailored to the individual patient based on the type of seizure and syndrome, comorbid medical, neurologic, and psychiatric disorders, concomitant medications, age, and socioeconomic factors (ability to afford newer AEDs). The unnecessary use of high doses of AEDs, such as phenytoin, is shown in Case 7, including the reported fatigue and rash, which is relatively more common in aromatic AEDs. Furthermore, in patients at risk for the development of a rash (e.g., those with a history of a rash to other drugs) the risk increases significantly when the drug is started at high doses.
Fortunately, a significant number of patients with new-onset epilepsy respond to AEDs started at low doses and titrated to a target dose of a period of several weeks. These include CBZ, lamotrigine, topiramate, oxcarbazepine (OXC), and levetiracetam. A very significant proportion of patients do not require intravenous loading and high doses of AEDs from the start. In fact, multiple double-blind, multicenter randomized studies have demonstrated the safety of starting patients with newly diagnosed epilepsy (either with primary generalized or partial epilepsy) on oral AEDs that require a titration over a period of time to reach an initial target dose. These trials included AEDs like CBZ, lamotrigine, topiramate, OXC, and levetiracetam.
Case 9. A 35-year-old man was brought to the emergency room after experiencing a cluster of three complex partial seizures in the last 24- hours. He had been started on CBZ 6-months ago and had remained free of seizures until this recurrence. A CBZ serum concentration of 7.5 mg/L was obtained. The patient did not report any adverse effects at this dose. The patient was advised to be started on another AED, as the CBZ serum concentration was within the “therapeutic range,” and hence, this AED would not be expected to make the patient seizure-free.
This is an extremely common error that results in unnecessary premature discontinuation of AEDs, which could yield a seizure-free state if the dose were to be adjusted to its true potential therapeutic effect, defined as the dose that gives the “best seizure control for this patient” in the absence of adverse events. In other words, testing of efficacy and tolerability of an AED must be based on its potential to yield seizure remission (or significant reduction of seizure frequency) at the maximally “tolerated” doses, independent of the serum concentration. Premature discontinuation of AEDs often leads to the false assumption of pharmaco-resistance. After all, the concept of a therapeutic range is based on a statistical observation but is not a reflection of the individual’s own tolerance to the AED. In fact, it is not unusual to find patients who become seizure-free at serum concentration below the therapeutic range, and conversely patients
able to tolerate doses with serum concentrations above the therapeutic range.
Case 10. A 55-year-old man was started on phenytoin because of a new-onset seizure disorder. He was loaded with 15 mg/kg of fosphenytoin in the emergency room after a third secondarily GTC seizure and was discharged on a regimen of 300 mg/day of brand phenytoin (Dilantin®) to be taken all at night. Three days after being discharged from the emergency room, he returned complaining of ataxia, diplopia, and nausea. A phenytoin serum concentration was 7 mg/L. Symptomatic treatment was suggested, and he was told that he probably had a flu-like syndrome and that he should see his family doctor the next day. Since his phenytoin level was 7 mg/L, he was reloaded with 10 mg/kg of fosphenytoin to bring his level back to 17 mg/L, and he was instructed to increase his maintenance dose of phenytoin to 350 mg/day. By the end of the IV infusion, the patient started to vomit and reported severe vertigo and diplopia and was unable to stand up and walk without assistance without falling to the side. He was admitted to the neurology service with a diagnosis of possible cerebrovascular accident in the territory of the posterior circulation. A stat CT scan of the brain was unremarkable. A hemogram was remarkable for mild microcytic anemia (hemoglobin, 10.8 g/dL) and a chemistry panel was remarkable for albumin of 2 g/dL. Given the low serum albumin concentration, the neurologist ordered a free phenytoin level, which came back at 3.8 mg/mL; he ordered the phenytoin to be held and 48 hours later, the patient was asymptomatic.
This case shows another very common error on dosing phenytoin. This AED is highly bound to albumin (90%). In cases of hypoalbuminemia or conditions that limit the albumin binding, such as uremic states, the free fraction of phenytoin increases while the total concentration decreases. Since the free fraction is the one that crosses the blood-brain barrier, a doubling of the “normal” free serum concentrations (i.e., from 10 to 20%) is equivalent to a “doubling” of the amount of phenytoin entering the central nervous system (CNS). Thus, in Case 10, the patient’s symptoms were the expression of phenytoin toxicity resulting from a free fraction of 3.8 mg/L equivalent to a total serum concentration of 38 mg/L.
The Sheiner-Tozer equation was developed to estimate the free fraction of phenytoin: estimated phenytoin levels = total phenytoin (mg/L) [(albumin g/L × 0.9) + 0.1]. It should be emphasized, however, that this equation gives an “estimated” measure, and blood should be drawn to obtain an accurate free serum concentration. This case also emphasizes the fact that phenytoin serum concentrations cannot be interpreted in a reliable manner without knowing the patient’s albumin serum levels. Furthermore, keep in mind non-pathologic conditions associated with “high” phenytoin free concentrations, such as that which occurs when phenytoin is given in combination with valproic acid or in elderly patients. In the former case, valproic acid has a higher affinity to albumin and displaces phenytoin from the albumin sites. Under those circumstances, the Sheiner-Tozer equation is not applicable and free phenytoin concentrations must be measured.
There is no question that seizure-remission is the first goal in the treatment of patients with epilepsy. Yet, in a significant number of patients, seizure disorders occur in the setting of underlying medical, neurologic, and psychiatric disorders that precede the onset of seizures, for which they are taking one or more medications. Failure to take into consideration the impact of the chosen AED on comorbid conditions and concomitant medications constitutes one of the more common errors in daily practice.
Case 11. A 39-year-old man with a history of frontal lobe epilepsy and major depressive disorder was brought to the emergency room with complaints of suicidal ideation consisting of thoughts of “wanting to jump out of the window.” Three weeks before, he had been discharged from the video-EEG monitoring unit where he had undergone a presurgical evaluation and at the end of which the AED he was on, OXC, was discontinued and he was switched to phenytoin. His mood disorder had been in remission for several years on a regimen of citalopram at a dose of 40 mg/day. The patient demanded to be put back on OXC, blaming his symptoms on the phenytoin. He was admitted for observation and his dose of citalopram was raised to 60 mg/day. Two weeks later, he was discharged home in a euthymic state.
This case shows a failure to factor in a 30 to 50% increased clearance of citalopram caused by the enzyme induction of phenytoin, which shares the same cytochrome P450 isoenzyme (CYP3A4) with citalopram. The synthesis (also known as induction) of this isoenzyme by phenytoin resulted in an increase in its metabolism and drop of its serum concentrations, which was responsible for the patient’s recurrence of symptoms of depression.
Ignoring the pharmacokinetic properties of AEDs and their impact on concomitant medication and “endogenous” substrates (i.e., hormones, vitamins, etc.) is a very common error in clinical practice. Thus, the addition or discontinuation of enzyme-inducing AEDs must be followed by the adjustment of doses of concomitant medications metabolized in the liver whose clearance may be increased by these AEDs, as failure to make these dose adjustments may have a negative impact on their efficacy resulting from a decrease in serum concentrations. The enzyme-inducing AEDs include phenytoin, CBZ, phenobarbital, primidone, and topiramate and OXC at doses greater than 400 mg/day and 1200 mg/day, respectively. Likewise, AEDs that inhibit the metabolism through inhibition or slowing of metabolic pathways like glucuronidation (valproic acid) or inhibition of isoenzymes (felbamate) are likely to cause toxicity of concomitant medications whose serum concentrations to increase as a result of such pharmacokinetic interaction. A review of pharmacokinetic interactions between enzyme-inducing AEDs and some of the commonly used concomitant medications and with endogenous molecules (hormones and vitamins) are reviewed in the next section.
CBZ and the two antidepressants citalopram and escitalopram are metabolized by common isoenzymes of the CP450 system (CYP2C19 and CYP3A4). It has been found that the plasma concentration of citalopram and escitalopram was lowered by 27% and 31%, respectively, after 4 weeks of CBZ therapy. By the same token, CBZ and the antidepressant mirtazapine are metabolized by the isoenzymes CYP2D6, CYP1A2, and CYP3A4. The area under the curve of mirtazapine was decreased by 63% when given to healthy volunteers in conjunction with CBZ. Phenytoin has been found to have the same impact on mirtazapine. In the case of sertraline, which is metabolized by the isoenzymes CYP2C19 and CYP384, the addition of CBZ resulted in a lack of efficacy at doses two to four times higher than the minimum effective dose. Finally, enzyme-inducing AEDs are known to increase the clearance of most typical and atypical antipsychotic drugs metabolized in the liver.
The pharmacokinetic interaction between enzyme-inducing AEDs and calcium channel blockers is also significant. For example, the concomitant use of enzyme-inducing AEDs that are metabolized by the CYP3A isoenzyme resulted in an 85% reduction of the area under the curve of nimodipine and 90% of the area under the curve of nisoldipine. OXC, which is a modest enzyme-inducing AED, resulted in a 30% reduction in the area under the curve of the calcium channel blocker felodipine.
Another common concomitant medication used in cardiac patients is warfarin. This drug is metabolized by the isoenzymes CYP3A4 and CYP2C9. Phenobarbital has been shown to increase the clearance of warfarin by 65%. CBZ was reported in several case series to decrease its anticoagulant efficacy, whereas the interaction between warfarin and phenytoin is complex with inhibition of the isoenzyme 2C9 by s-warfarin and induction of the isoenzyme CYP3A4 resulting in increase or decrease in the anticoagulant effects.
Such interactions can have catastrophic effects on patients who are receiving chemotherapy. For example, in a study of 716 children with acute lymphoblastic leukemia, 40 children who were on enzyme-inducing AEDs were found to have worse event-free survival, hematological relapse, and CNS relapse. These children were found to have a higher clearance of teniposide and methotrexate. In a separate study of 9 patients on CBZ or phenytoin and 6 patients on AEDs that were not enzyme-inducing, the impact of the AEDs was studied on the metabolism of vincristine given for the treatment of brain tumors. The clearance of vincristine in patients on CBZ and phenytoin was 63% higher, its half-life 35% shorter, and its area under the curve 43% smaller.
Clearly, the use of enzyme-inducing AEDs carries significant negative impact in the efficacy of concomitant medications metabolized by shared isoenzymes; hence, the dose of these concomitant medications has to be adjusted when enzyme-inducing AEDs are introduced.
The pharmacokinetic interaction of enzyme-inducing AEDs with concomitant medication has a significant negative impact in elderly patients, as this is a patient population with relatively frequent comorbid medical conditions and therefore a significant number of other medications.
As mentioned above, the impact of enzyme induction of a drug is not restricted to concomitant medications, but also to endogenous molecules, such as hormones and vitamins, resulting in pathologic conditions. For example, osteoporosis is a condition that is strongly suspected to be associated with the use of enzyme-inducing AEDs. This condition can have very significant implications for a patient population like that of people with epilepsy, as this is already a population at increased risk for suffering from fractures that may be related to trauma caused by the epileptic seizures, as well as falls associated with AED-related toxicity such as ataxia, diplopia, dizziness, or blurred vision. In elderly patients, the development of hip fractures can have an extremely negative impact with respect to increased mortality of up to 20%.
The mechanism by which enzyme-inducing AEDs facilitate the development of osteoporosis remains controversial. Some studies have suggested that a decrease in the bone mineral density results from an increase in the metabolism of vitamin D, which then interferes with the absorption of calcium. Another mechanism proposed suggests an increased bone osteoclastic activity mediated by high parathyroid hormone levels, triggered in turn by calcium levels or a combination of all of the above. Unfortunately, studies have shown that adult and pediatric neurologists do not screen for bone health problems in patients taking AEDs.Few neurologists (9% of pediatric and 7% of adult neurologists) prescribe prophylactic calcium or vitamin D for patients taking AEDs.
Enzyme-inducing AEDs facilitate the synthesis of sex hormone-binding proteins which decrease the availability of free estrogen and testosterone serum concentrations. This, in turn, has been associated with a decreased libido that is very prevalent among patients with epilepsy. This is an area that in general remains neglected by most clinicians who don’t investigate the sexual lives of patients with epilepsy. Yet, decreased libido has been shown to impact negatively on the quality of life of these patients. Patients with epilepsy are characteristically known for having a lower number of children, even when they are married, which has also been attributed to decreased libido.
Common Errors in the Surgical Treatment of Epilepsy
Case 12. A 42-year-old man with a 20-year history of TLE was referred for presurgical evaluation. A brain MRI revealed right hippocampal atrophy. During his video-EEG monitoring, 95% of interictal spikes and four complex partial seizures were recorded from the right anterotemporal region. Neuropsychological testing revealed impaired memory for visual-spatial delayed memory. His seizures had been treated with eight AEDs given in monotherapy and polytherapy regimens up to subtoxic doses. He never went for more than 3-weeks without seizures. He felt frustrated by the fact that he had not been referred earlier for epilepsy surgery, as he felt he could have gone to college, had he had surgery when he was younger.
This case shows a very common error, mainly a failure to recognize a pharmacoresistant seizure disorder in its early stages and an unnecessary delay in the referral for presurgical evaluation that can identify patients with a 50 to 80% probability of achieving a seizure-free state with surgical treatment. Indeed, among all patients with epilepsy, 30 to 40% are expected to suffer from a seizure disorder that will fail to remit with AEDs. Seizure disorders of focal origin that fail to remit after three trials with the appropriate AED at optimal doses can be considered to bepharmacoresistant. Some investigators have suggested that pharmacoresistance can be considered after two unsuccessful AED trials.
In patients with partial seizure disorders, epilepsy surgery has become an alternative treatment that can yield seizure freedom in 50 to 80% of patients that are considered to be good candidates. Seizure freedom has consistently been found to occur in 60 to 70% of adults and 65 to 80% in children following temporal lobectomy. Extratemporal resections less commonly lead to a seizure-free outcome, although some childhood series have reported a seizure-free rate of 60% following extratemporal epilepsy surgery. Furthermore, in patients whose seizure disorder fails to remit with epilepsy surgery, both temporal and extratemporal resections yield a significant reduction in seizure frequency in a significant percentage.
Suspicion of pharmacoresistance can be heralded after an unsuccessful AED trial at optimal doses in patients with certain types of epilepsy.
The identification of pharmacoresistant epilepsy is possible at relatively early stages of the patients’ disease. Unfortunately, the time from onset of the epileptic disorder to the time of referral for presurgical evaluation hovers between 15 and 20 years in most of the surgical series. Such delay is unacceptable today. First, an unsuccessful AED trial (defined as persistent seizures at optimal doses) can be demonstrated within a period of 3 and at the most 6- months in a majority of patients. Thus, identification of seizure disorders that are pharmaco-resistant can be achieved over a period of 12 to 18 months in most patients. To that end, a dose escalation schedule should be implemented if patients continue experiencing seizures after reaching a target dose for a period of at least 4 weeks and continue increasing the dose of the AED at hand until seizure freedom is achieved or adverse events are reported, whichever occurs first. Once the first drug has been shown to be ineffective, the patients should be switched to a second AED to be ultimately given in a monotherapy regimen following the same process as with the previous AED trial. If unsuccessful, a third AED trial should be considered, and if seizures persist, the patient should be referred for presurgical evaluation.
Second, high-resolution brain MRI studies have facilitated the identification of those structural lesions associated with poor response to pharmacotherapy (i.e., MTS and malformations of cortical development such as focal dysplasias). Unfortunately, these types of lesions go often undetected with “standard” MRI studies. Thus, neurologists must ensure that they are ordering high-resolution MRI studies in any patient whose seizures have a clinical semiology suggestive of TLE or in patients whose seizures have failed to remit with at least one AED trial at optimal doses.
Failure to Evaluate and Treat Comorbid Psychiatric or Neuropsychological Conditions
Case 13. A 45-year-old woman was admitted to the psychiatry floor after attempting to commit suicide with an overdose of her AEDs. This was the third attempt in 3 years. During previous admissions, the patient had been started on an antidepressant and advised to follow up in the outpatient psychiatry clinic. However, she never followed the recommendations, giving the excuse that her neurologist never insisted that she see a psychiatrist. She would usually take the antidepressant for 1 or 2 months and then stop it on her own. She had learned to taper down the dose over a period of 2-weeks to avoid withdrawal symptoms.
She stated that “she has been depressed for as long as she can remember” and probably before she started to suffer from epileptic seizures 30-years ago. Her mother suffered from depressive episodes as well, and her father was an alcoholic. She described her depressive episodes as short and long ones. The short episodes would typically appear 2-days after her seizures and last for a period of 3 to 7 days. During these periods, she would experience a worsening of her mood and would become very irritable, after which she would feel very guilty. She would be unable to find any pleasure in any activity, had marked difficulty concentrating, and would lose her appetite. She would be able to sleep only for 2 or 3 hours at a time during these episodes. Occasionally she would experience suicidal ideation which she would try to ignore, as she knew those thoughts would be gone in a few days.
The longer episodes would be similar in the type of symptoms but would last between 2 and 8-weeks, and in between them, she would never be back to a state of “normal mood.” She stated that her mood disorder was worse than her complex partial seizures with an average frequency of four to six per month, at times in clusters of three seizures over 2-days. She rarely had GTC secondarily seizures. After a presurgical evaluation, she was deemed to be an unsuitable candidate because of seizures originating from both temporal lobes.
This case illustrates another frequent error, mainly the failure to recognize and treat comorbid psychiatric disorders, specifically attention deficit disorder (ADD) and depressive and anxiety disorders. Failure to provide treatment for such conditions has a significant negative impact on the quality of life for these patients. In the case of mood disorders, several studies have identified depression as the strongest predictor for the poor quality of life.
Failure to treat these patients is often based on the misunderstanding that the use of antidepressant medications can worsen seizures. In fact, there is now strong evidence that the lowering of the seizure threshold by antidepressant medication occurs only at toxic doses and with four specific antidepressants, three of which are rarely prescribed today. These are clomipramine, maprotiline, amoxapine, and bupropion when given in their immediate-release formulation at doses higher than 300 mg/day. Among these, bupropion is the only one that is prescribed on a regular basis by clinicians but should be avoided in patients with epilepsy. Serotonin-reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been found to be safe. Recent studies done in animals have demonstrated that serotonergic and noradrenergic neurotransmissions have anticonvulsant effects in animal models of epilepsy. Thus, antidepressant medication would, in theory, have an antiepileptic effect in humans.
Failure to recognize and treat comorbid psychiatric disorders is not limited to mood and anxiety disorders. Indeed, clinicians often fail to recognize and treat the presence of a comorbid ADD in patients with epilepsy. The prevalence of ADD in children with epilepsy, especially those with poorly controlled seizures, has been estimated to range between 30% and 50%. The prevalence in adults is yet to be established, which is a reflection of the complete failure to screen for this disorder in this age group. Children with ADD without hyperactivity have been found to be two and a half times more likely to develop epilepsy.
Despite the extensive use of CNS stimulants in patients with epilepsy, many physicians still prefer not to treat ADD pharmacologically because of a long-held misconception that these drugs worsen seizures. Unfortunately, this misconception is found in the Physicians’ Desk Reference manual. The data are based on anecdotal reports. In fact, several studies have failed to demonstrate a lowering of the seizure threshold in patients with and without epilepsy. It is likely that the seizure occurrence identified in children with ADD treated with CNS stimulants may reflect the increased risk of developing epilepsy and is not related to the drug itself. In patients with refractory epilepsy, exposure to CNS stimulants does not worsen the seizure frequency.
The lack of data on the prevalence of ADD in adults with epilepsy is probably related to the remission of motor hyperactivity when children reach adolescence. Nonetheless, between 50% and 75% of children with primary ADD are expected to remain symptomatic with problems of attention, impulsive behavior, and poor frustration tolerance. Whether such is the case in adults with epilepsy remains to be established. Unfortunately, these patients are likely to be at increased risk to fail academically and in their work as well as to have problems in their social life because of their impulsivity, irritability, and poor frustration tolerance.
In previous years, ADD was associated with partial seizure disorders. In fact, recent data indicate that children with primary generalized epilepsy are also at increased risk of suffering from ADD. Trials with CNS stimulants, such as methylphenidate, or their extended-release formulations are typically recommended.
Finally, cognitive comorbid disturbances not necessarily associated with ADD that go unrecognized and unattended are encountered in children and adults with epilepsy. It is therefore essential that any child with epilepsy be screened for a potential learning disability that may be interfering with his or her ability to learn.

Leave a Reply
Want to join the discussion?Feel free to contribute!