Perioperative Peripheral Nerve Injury
The purpose of this review is to comprehensively review perioperative peripheral nerve injury (PNI) associated with general anesthesia and discuss the epidemiology, mechanism of injury, intraoperative monitoring, and prevention.
Perioperative PNI is an important cause of disability and malpractice claims. However, the multifactorial etiology of PNI may not be appreciated in malpractice claims, with PNI often alleged to be related to errors in patient positioning on the basis of res ipsa loquitur (“the thing speaks for itself”).
New advances in monitoring may aid in the early detection of PNI. Recent automated detection technology in neuromonitoring with somatosensory evoked potentials may increase the ability to identify at-risk patients and individualize patient management.
Anatomy of Peripheral Nerves
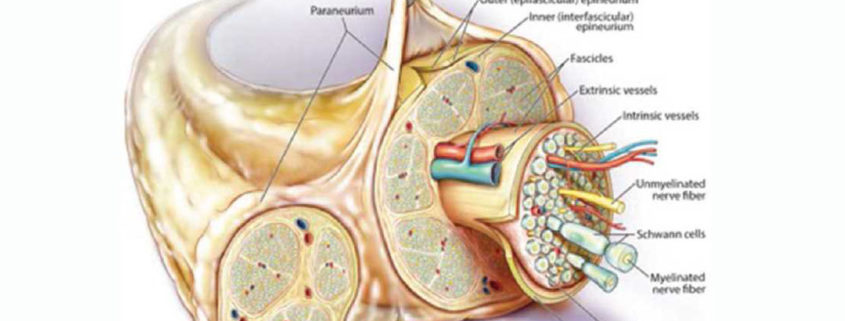
Peripheral nerves are composed of bundles of parallel nerve axons (or fibers), ranging from 0.3 to 22 μm, embedded within multilayers of connective tissues (See image above). The endoneurium wraps around the axons and is bundled into fascicles by the perineurium. For myelinated fibers, Schwann cells invaginate the nerve fibers in multiple layers to form an insulated membrane (“myelin sheath”) to enhance impulse conduction.
A peripheral nerve is a metabolically active tissue that requires an uninterrupted blood supply to maintain its cellular integrity and function, which is supplied only by a delicate capillary network in the endoneurium, the vasa nervorum. Because peripheral nerves consist of a series of cellular components and supporting tissues with variable blood supplies, it is possible to have areas of local ischemia without involving the entire peripheral nerve.
Definition and Classification of PNI
One challenging aspect is the multitude of terms used to describe PNI. These terms, including “nerve insult,” “neurapraxia,” “nerve injury,” “neuropathy,” and “nerve dysfunction,” are often misused and used interchangeably, resulting in significant miscommunication and misinterpretation of available evidence.
The term “PNI” means a severe nerve insult that results in loss of anatomical nerve integrity. PNI is commonly classified using either the Seddon or Sunderland classification, in which the severity of the injury is based on the degree of axonal and glial damage. In the Seddon classification, the severity of nerve injuries ranges from segmental loss of myelination (neurapraxia), through loss of individual axons (axonotmesis), to the destruction of the entire nerve trunk (neurotmesis). Neurapraxia is often used to describe perioperative PNI because most perioperative PNI patients recover shortly after surgery. However, this description is misleading.
The term “nerve insult” is a descriptor of a damaging event that potentially leads to injury, but it does not convey the severity of the injury. Therefore, “nerve insult” encompasses a continuum from transient dysfunction with no structural damage to permanent loss of the integrity of the peripheral nerve. In mild nerve insult, the nerve conduction is impaired (often called “neuronal dysfunction”), but the nerve is structurally intact. This change in conduction is a physiological response to nerve insult and can be detected by intraoperative neurophysiological monitoring (e.g., somatosensory evoked potential [SSEP] or motor evoked potential monitoring). Therefore, an abnormal signal change during intraoperative neurophysiological monitoring only represents nerve conduction characteristics at that particular time and may not be associated with actual structural nerve damage. Patients with severe nerve insult resulting in structural damage are described as having PNI and the severity of structural damage further subclassified using either the Seddon or Sunderland classification.
In the American Society of Anesthesiologists (ASA) Practice Advisory, perioperative peripheral neuropathy is defined as “postoperative signs and symptoms related to PNI (e.g., brachial plexus, sciatic, and femoral). Symptoms may include but are not limited to paresthesias, muscle weakness, tingling, or pain in the extremities.” In neurology, peripheral neuropathy is often used to imply a more chronic or permanent condition. In the context of perioperative PNI, most authors refer to perioperative peripheral neuropathy as having occurred in patients who have a new onset of postoperative signs and symptoms related to PNI that persist for at least 4–6 weeks. For the purposes of this article, PNI will be defined as a severe nerve insult that results in loss of anatomical nerve integrity, manifest by symptoms and signs including, but not limited to, numbness, paresthesia, tingling, pain, or muscle weakness.
Incidence of Perioperative PNI
The incidence of perioperative PNI after general anesthesia varies across different surgical procedures. Cardiac surgery, neurosurgery, orthopedics, and general surgery seem to be associated with higher risk of PNI. Hypertension, diabetes, and smoking history were significant risk factors for perioperative PNI.
Some surgical procedures report substantially higher PNI incidences, such as shoulder surgery (4.3%–8.2%) and total hip arthroplasty (0.72%), implying a different injury pattern. PNI after cardiac surgery (incidences of 0.8%–6.1%), appears multifactorial and it is difficult to distinguish causality whether anesthetic- or surgically related, or other.
Specific PNI
Ulnar PNI
The ulnar nerve may be anatomically susceptible to injury because it transverses the ulnar groove, a closed and tight space formed by the aponeurotic band between the medial epicondyle and the olecranon. Men may be anatomically more susceptible to injury because the tubercle of the coronoid process is approximately 1.5 times larger than in women.
Brachial Plexus Injury
The brachial plexus is susceptible to stretch and compression injuries. There are 2 clear patterns of brachial plexus PNI: (1) classic postoperative brachial plexus injury and (2) poststernotomy brachial plexus injury. Classical brachial plexus PNI occurs during hyperextension of the shoulder such as with steep head-down position. Brachial plexus PNI after cardiac surgery is associated with retractors and surgical technique, significant perioperative physiological changes, inflammatory responses, and patients with multiple risk factors. Thus, cardiac surgical patients are particularly vulnerable to intraoperative insults and “double crush” injury, as discussed below.
Lower Limb PNI
Injuries to the peripheral nerves of lower extremities most commonly involve the common peroneal nerve, sciatic nerve, the lateral cutaneous nerve of thigh, and obturator nerve. Direct mechanical injuries may occur in lithotomy position due to overstretching of the sciatic nerve by external rotation and flexion of the hip joint or direct pressure from the hard operative table. Similar to poststernotomy brachial plexus injury, intraoperative ischemic injury, systemic inflammation, and a higher intrinsic patient susceptibility may be potential causes.
Mechanisms of PNI
Understanding the causes of PNI is important for proper risk evaluation and injury prevention. A long-standing assumption is that perioperative PNI is caused by direct mechanical insult. Many legal claims of PNI invoked the doctrine of res ipsa loquitur, inferring that PNI could not happen without negligence of the caregivers. Despite the fact that some cases of PNI occur without an identifiable cause and despite application of standard preventive measures (e.g., patient appropriately padded and positioned), anesthesiologists have often been cited for negligence.
In many instances, PNI may be multifactorial, caused by a combination of local (e.g., stretching, compression, ischemia, and transection) and systemic (e.g., systemic hypotension and inflammation) insults. The manifestation of PNI depends on the “dose” of injury (i.e., severity and duration of nerve insult) and the underlying neuronal reserves which are impaired with preexisting peripheral neuropathy (discussed below).
Local Mechanical Insult
Stretching and compression are the 2 most common local mechanical insults resulting in position-related PNI, whereas transection may occur in needle-related injury during regional anesthesia or by direct surgical-related injury. Both stretching and compression may result in direct mechanical deformation of peripheral nerves and ischemic injury. Stretching of a peripheral nerve by only 5% may cause it to begin to kink and/or collapse its feeding arterioles or venules, resulting in ischemia and venous congestion. Ischemic injury induces increased permeability of epineurial vessels, leading to intrafascicular edema and increases the intraneural pressure further reducing intraneural perfusion pressure, producing secondary injury to the peripheral nerve. When the axon is lacking an extensive vascular supply, it is more susceptible to stretch-related injury. An animal study found that a nerve stretch of 6% for 1 hour produced a reversible 70% reduction in action potential while a stretch of 12% resulted in permanent injury. Examples of stretch-related perioperative PNI include hyperabduction of the shoulder in the prone surrender position (the prone position with both shoulders 90° abduction, both arms at 90° flexion, and both arms in pronation) and sternal retractor–related brachial plexus injury in cardiac surgery.
Compression can cause mechanical deformation, or interruption of perfusion (ischemic insult), and subsequent injury to the peripheral nerve. To date, there has been no quantitative study that has assessed the relationship between compressive force and injury. Susceptibility to direct pressure on unmyelinated ulnar nerve fibers is greater in men than in women. A typical example of compression-related injury is ulnar neuropathy in patients with a supinated arm on a rigid arm-board, or “Saturday night palsy” (radial nerve injury resulting from falling asleep with one’s arm hanging over the armrest of a chair) manifesting as wrist drop, hand and finger numbness, and inability to straighten the fingers due to prolonged compression of the radial nerve.
Ischemic Insult
Intraneural hypoperfusion and ischemia appear as a common final pathway of local and systemic insults. Ischemia induces a vicious cycle of tissue edema, disruption of cellular integrity, increased intraneural pressure, and further reduction of intraneural perfusion pressure which can result in necrosis. Clinical examples are compartment syndrome or during systemic hypotension.
In a study by Swenson et al., direct occlusion of the brachial artery at the elbow level caused rapid (<4 minutes) abnormal SSEP changes. The ulnar nerve had greater and earlier SSEP amplitude reduction than the median and radial nerves. This study suggested that peripheral nerves are very sensitive to ischemic insult and special care should be taken to ensure adequate perfusion. A case-control study in the prone surrender position also found an association between systemic hypotension and abnormal SSEP. Sustained intraoperative hypotension (mean arterial pressure <55 mm Hg) for as little as 5 or more minutes was associated with an increased risk of developing abnormal SSEP compared with transient (<5 minutes) intraoperative hypotension. Maintaining high systematic blood pressure (mean arterial pressure >80 mm Hg) throughout the surgery was found to be protective against developing abnormal SSEP.
Inflammation/Metabolic
More recently inflammatory and autoimmune mechanisms have been identified as causes for PNI. Staff et al. examined nerve biopsies from 23 patients who presented with pain and muscle weakness within 30 days after surgery and found that 21 had abnormal pathological features of either increased epineurial perivascular lymphocytic inflammation or microvasculitis. No other mechanical causes were identified in these patients. Some cases were improved with immunotherapy (methylprednisolone and/or intravenous immunoglobin). Another case series of 7 patients with ipsilateral weakness after hip surgery reported similar findings with nerve biopsies showing ischemic and inflammatory injuries to the microvessel wall and axonal degeneration; 6 patients were treated with intravenous methylprednisolone, and all had favorable clinical improvement. This subset of PNI which develops after surgery without an identifiable operant cause and which is potentially treatable with immunotherapyis described as postsurgical inflammatory neuropathy.
It has been observed that many patients with PNI developed symptoms more than 48 hours after surgery, especially those with upper extremity neuropathy, while the patients who are bedbound or faced with prolonged immobility in medical units developed ulnar neuropathies similar to those in surgical units. Combined, these studies suggest that postoperative causes such as inflammation and prolonged immobilization are important contributors to perioperative PNI.
Neuronal Reserve: Double Crush Syndrome
The underlying neuronal function is impaired in patients with preexisting neuropathy and is an important factor in PNI, as many patients with marginal underlying neuronal reserve experience a worsening of their symptoms during the perioperative period. This phenomenon can be partly explained by a secondary injury at the lesion site as explained by the model of “double crush” injury.
Double crush syndrome occurs when 2 distinct subclinical nerve insults occur, producing synergistically detrimental effects that result in clinically apparent neuropathy. In a nonperioperative setting, double crush injury has been well described in patients with carpal tunnel syndrome and a secondary lesion such as thoracic outlet obstruction, cervical radiculopathy, or ulnar neuropathy. Patients with double crush injuries in the nonperioperative setting were found to have poorer patient-reported outcomes, less nerve recovery, and lower satisfaction after carpal tunnel release. In the perioperative setting, especially after cardiac surgery, double crush injury is often invoked in relation to the onset of perioperative PNI. The exact mechanism is unknown, and the types of underlying neuropathy that are able to reliably predict double crush injury have yet to be determined. The fact that risk factors of perioperative PNI (e.g., hypertension, diabetes, and tobacco use) are similar to risk factors for chronic microvasculopathy and neuropathy supports the hypothesis that underlying nerve function is a major determinant of perioperative PNI.
Medicolegal Risk of Perioperative PNI
Patients with PNI are more often male, adults, healthy, and having elective procedures compared to patients in claims for other complications. While mean age does not differ, patients in PNI claims are more likely to be adults (99%) than patients in other claims. Spine, nonspine orthopedics, cardiac, and neurosurgery procedures account for 39% of PNI claims, identical with other claims. Most (84%) PNI claims involve an upper extremity with 36% brachial plexus, 30% ulnar nerve, 10% median nerve, and 8% radial nerve.
The severity of injury is measured on a 9-point scale from 0 (no apparent injury) to 9 (death), collapsed into categories of temporary (score 0–4), permanent nondisabling (score 5), or permanent disabling and death (score 6–9). The majority of PNI are temporary (39%) or permanent nondisabling (37%). PNI claims are more likely to be permanent nondisabling and less likely to be disabling or fatal than injuries in other claims.
Prevention of Perioperative PNI
Current preventative strategy for perioperative PNI focusing on proper patient positioning, padding, and periodic assessment are important but remain insufficient to completely prevent perioperative PNI. Most recommended practices are based on consensus or low level of evidence. Many commonly used practices may be improperly executed and might result in failure or even paradoxical injury (such as padding the elbow too tightly).
As local mechanical insult is not the only contributor to perioperative PNI, other types of insults (e.g., sustained hypotension, inflammation) and preexisting neuronal function are often overlooked. Therefore, a simple preventative measure that is effective in all patients is unlikely to be found. Prevention may require a different strategy, such as continual monitoring of nerve function in selected high-risk patients and procedures.
Intraoperative Neurologic Monitoring
SSEP has long been used in neurology as a diagnostic test for peripheral neuropathy. In the surgical setting, SSEP is used to assess the completeness of nerve repair, to guide peripheral nerve decompression, and to detect spinal cord injury and cerebral ischemic injury in neurosurgical procedures.
SSEP is also used for early detection of peripheral nerve ischemia related to positioning in patients. Most studies found an association between abnormal SSEP and positioning and demonstrated that if SSEP changes were reversed by adjusting arm position, postoperative PNI did not subsequently develop.
One prospective study during cardiac surgery also found a high proportion (23/30 patients) of abnormal SSEP and PNI. Cardiac surgery patients with persistent (until the end of surgery) and severe (>3 SD) abnormal SSEP progressed to PNI, suggesting that there may be a “dosing effect” predisposing to PNI and that SSEP may be a reliable monitor to predict PNI.
SSEP, motor evoked potential, or electromyography (EMG) was assessed for detection of surgical-related nerve injury during total shoulder arthroplasty, shoulder rotator cuff repair, humerus fracture reduction surgery, and shoulder arthroscopy. These studies reported high incidences of signal changes, implying a high burden of nerve insults.
The use of SSEP monitoring for intraoperative detection of PNI is appealing as most perioperative nerve insults are potentially reversible and treatable. However, the diagnostic accuracy of SSEP specific for PNI is yet to be determined, and it is also unclear if SSEP can decrease the risk of developing PNI.
Postoperative Diagnosis and Management
Nerve conduction studies (NCS) and needle EMG are the key postoperative diagnostic tests in PNI. These tests confirm the diagnosis, localize the injury site, determine the injury degree, predict prognosis, and possibly direct surgical intervention. The timing of the NCS or EMG depends on the clinical questions under investigation. In the first 7 days after the injury, the NCS can localize the injury by identifying the site on a peripheral nerve where abnormal conduction is occurring. Typically, conduction proximal to the lesion is lost, whereas the distal conduction is preserved resulting in similar NCS and EMG findings regardless of the degree of injury.
If the NCS is deferred until 3–4 weeks after injury, patients with neurapraxia usually have normal results due to remyelination. In patients with axonotmesis and neurotmesis, both proximal and distal conduction is lost due to Wallerian degeneration. With extensive injury, the EMG shows fibrillation potentials and recruitment of motor-unit potential. Features of early axonal regeneration might be observed in patients with neurapraxia or less severe axonotmesis. Thus, electrodiagnostic tests at 3–4 weeks after surgery can distinguish the severity of PNI. A follow-up study at 3–6 months provides information regarding reinnervation. Many neurologists withhold the investigation until 3–4 weeks after the injury because, at this time, most diagnostic and prognostic information can be obtained in a single study.
Early NCS performed within the first week after nerve injury has several implications for malpractice claims. Localization of the lesion is fundamental to distinguish between anesthetic or position-related injury and direct surgical injury that may be detected by NCS. However, in some cases, early NCS may find only diffuse nonspecific slowing, which is not contributory for localization or delineating the mechanism of injury. Approximately one-third of malpractice claims (39%) involved patients who had a temporary injury. This implies that determining the location of the lesion in patients with even transient nerve dysfunction may still be important for medicolegal purposes. Consequently, the incidence of PNI is likely underestimated.
Patients affected by PNI might sustain long-term disability from motor deficits or chronic pain. The evidence concerning long-term disability varies across case mix and over time and is difficult to quantify. Surgical intervention is rarely required in perioperative PNI. Immunotherapy (methylprednisolone and/or intravenous immunoglobin) has also been reported as treatments for postsurgical inflammatory neuropathy, but their therapeutic effectiveness in treating perioperative PNI remains unproven.
New Developments in Intraoperative Monitoring
Routine clinical application of SSEP monitoring is currently limited due to large machine size, need for a designated technician and/or neurophysiologist, and a nonintuitive user interface. There is a recent report of using an automated SSEP technology for detection of PNI in 33 cardiac surgery and 21 total shoulder arthroplasty patients. This automated device consists of a small control box, a Bluetooth-enabled tablet, stimulation and acquisition cables, and nonneedle stimulation and acquisition surface electrodes. This device uses a novel proprietary algorithm for SSEP signal acquisition and optimization and a new automated electrocautery suppression technology for artifact rejection, which largely precludes the need for troubleshooting the technical issues during monitoring. An automated signal interrogation algorithm computes and updates the latency and amplitude content of each SSEP signal relative to baseline and signals an alert when relative amplitude decreases by 50% or latency increases by 10%. The device requires the attachment of 6 surface electrodes to perform SSEP monitoring and continuously stores all SSEP signals enabling report generation and further post hoc analysis.
Pilot studies reported favorable quality of the raw signals and the ease of technical set-up and data interpretation. Abnormal SSEP was detected in 15% of the 33 SSEP-monitored cardiac surgical patients, 3 of whom demonstrated prolonged signal changes (>1 hour) and 2 of whom developed symptomatic PNI confirmed with NCS.
These studies signify that the practical barriers of high-cost complicated logistics and requisite expertise for performing routine SSEP monitoring may have been largely overcome. However, use of automated SSEP for detection of intraoperative PNI still has several limitations. The current diagnostic criteria for abnormal SSEPs is largely based on data derived from detection of spinal cord injury where direct surgical trauma to the nerve results in permanent damage. Such all-or-none approach ignores the “dosing effect” where the duration and severity of insult are influenced by underlying neuronal reserves and may be ameliorated by early detection and prevention. However, there are no studies demonstrating prevention of perioperative PNI (not just nerve ischemia) by performing SSEP monitoring.
Future Research
Perioperative inflammation appears to be a potentially important mechanism of PNI that requires further investigation. The unresolved questions include when inflammation occurs, how to identify patients with inflammatory injury and the type and duration of immunotherapy that should be used. As perioperative PNI is a combination of multiple intraoperative nerve insults, it is important to explore how these might be mitigated by early intraoperative detection and how they interplay with individual patient factors.

Leave a Reply
Want to join the discussion?Feel free to contribute!