Erb’s Palsy Lawsuits
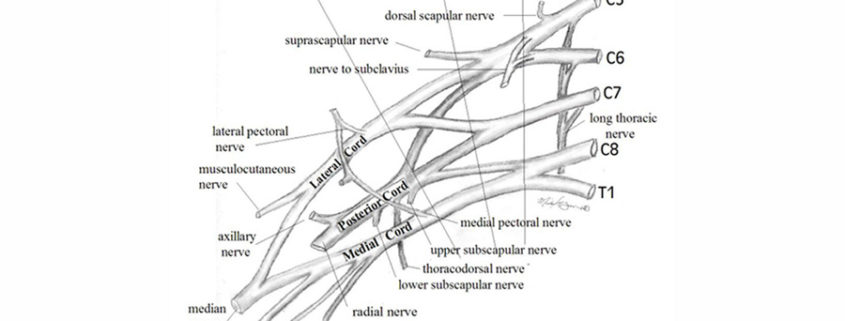
To understand the clinical presentation of brachial plexus palsy (BPP) and provide anticipatory guidance for families affected by the condition, the clinician must first know basic anatomy. As seen in the image above, the brachial plexus consists of nerves (the ventral rami) from C5-T1.
C5 and C6 join to form the upper trunk, C7 travels alone as the middle trunk, and C8-T1 join as the lower trunk. Each trunk divides into anterior and posterior divisions to create the cords, which then subdivide further into branches that supply the muscles of the arm. Injuries of the brachial plexus may be mild, with only temporary sequelae, or devastating, leaving the child with a flaccid, insensate arm.
Severity depends on the number of nerves involved and the degree to which each level is injured. The basic types of BPPs include the following:
- Erb’s palsy affects nerves arising from C5 and C6.
- Upper-middle trunk BPP involves nerve fibers from C5, C6, and C7 levels.
- Klumpke palsy results in deficits at levels C8 and T1, although many clinicians agree that pure C8-T1 injuries do not occur in infants and may be indicative of spinal cord injury (SCI).
- Total BPP affects nerves at all levels (C5-T1).
- Bilateral BPP demonstrates bilateral involvement.
When defining the severity of a peripheral nerve injury, differentiation between neurapraxic, axonotmetic, and neurotmetic lesions is helpful.
- Purely neurapraxic lesions do not affect the axon itself. These lesions generally are reversible and do not leave sequelae.
- Axonotmetic lesions involve disruption of the myelin sheath and the axon, leading to degeneration of the axon distal to the injury. The connective tissue across the lesion remains intact. These injuries improve gradually over 4-6 months, depending on the level of the lesion.
- Neurotmetic lesions are the most severe, destroying not only the axon and myelin, but also the supporting structures across a nerve. As the proximal end of the nerve attempts to regenerate without this supportive connective tissue, a neuroma may develop. The extent of improvement in the patient’s condition depends on the ultimate number of nerve fibers that reconnect distal to the neuroma. Muscle atrophy from a neurotmetic lesion begins 3-6 months after injury and by 1.5-2 years is irreversible.
Although the traditional mechanism of injury is lateral neck flexion, the upper rootlets (C5-C7) are 25% as likely to be avulsed as the lower roots (C7-T1). The upper roots (C5-C6), however, are far more likely to be ruptured (88%) because of the anatomy of the transverse processes and the degree of flexibility at that level.
The clinician must also distinguish neonatal BPP from traumatic BPP in older children and adults. The damage in neonates usually results from slow traction injuries, unlike the high-energy shearing type of trauma seen in older individuals. Not only are the latter injuries often more severe, but with similar injuries, infants show a better functional outcome.
Frequency in the United States
An incidence of 0.5-4.4 cases of brachial plexus palsy per 1000 full-term births has been reported; however, a review by Gilbert reported an incidence of 0.8-1 case per 1000 births.
Mortality/Morbidity
The incidence of permanent impairment is 3-25%.
The rate of recovery in the first few weeks is a good indicator of the final outcome. Complete recovery is unlikely if no improvement is noted in the first 2 weeks of life.
Age
Neonatal brachial plexus palsy is noted at birth.
History
When an infant is born with abrachial plexus palsy, the condition generally is apparent from birth. In a common scenario, the baby weighs over 4 kilograms and is the product of a difficult delivery to a multiparous woman, requiring the use of vacuum extraction or forceps. Upon delivery, which may involve anterior shoulder dystocia, the arm hangs loosely at the child’s side. Respiratory depression may indicate anassociated phrenic nerve palsy.
Physical
Newborn findings
The infant with complete brachial plexus palsy (BPP: C5-T1) typically lies in the nursery with the arm held limply at his/her side. Deep tendon reflexes (DTRs) in the affected arm are absent, and the Moro response is asymmetrical, with no active abduction of the ipsilateral arm.
In children with total arm involvement, careful examination of the child’s eye often demonstrates Horner’s syndrome (i.e., miosis, ptosis, anhidrosis), suggesting injury to the stellate ganglion.
Children with intrinsic hand weakness associated with BPP generally have Horner’s syndrome, and vice versa.
Respiratory status should be evaluated, since the phrenic nerve can be injured simultaneously.
The infant with an upper plexus palsy (C5-C7) keeps the arm adducted and internally rotated, with the elbow extended, the forearm pronated, the wrist flexed, and the hand in a fist. In the first hours of life, the hand also may appear flaccid, but strength returns over days to months.
The right side is injured in 51% of cases. Left BPP occurs in 45% of patients and bilateral injuries, in 4%.
The infant with a nerve injury to the lower plexus (C8-T1) holds the arm supinated, with the elbow bent and the wrist extended.
Sensation should be assessed closely, with the clinician noting any sensory loss in corresponding dermatomes.
Reflexes, typically absent in the affected limb, should be evaluated. This examination is particularly important in distinguishing BPP from hemiparesis, where reflexes may be brisk. Reflexes do not typically return in BPP except in the mildest injuries.
In the newborn nursery, it is essential that the physician carefully inspect the size of the hand and arm and the bulk of the pectoralis major muscle, along with palmar dermatoglyphics and limb range of motion (ROM), looking for clues indicating when the injury occurred. On occasion, injuries occur during gestation. In these cases, a child may, at the time of delivery, already have a smaller limb with asymmetrical palmar creases, pectoralis muscle atrophy, and/or joint contractures.
Associated injuries
The pediatrician must perform a careful examination of the infant with a BPP to look for associated injuries.
The most common associated (not causative) injuries include the following:
- Clavicular and humeral fractures
- Torticollis
- Cephalohematoma
- Facial nerve palsy
- Diaphragmatic paralysis
Findings in older children
The root level(s) and severity of injury ultimately determine the clinical picture and, in part, the outcome as a child ages.
The older child with BPP involving the upper trunk typically has difficulty with active shoulder abduction, forward flexion, symmetrical elbow flexion, and forearm supination.
With shoulder abduction, the medial edge of the scapula often can be seen protruding above the shoulder line, a manifestation referred to as Putti sign.
The reduction in shoulder abduction is due in part to weakness of the deltoid and in part to the lack of external rotation, which is needed for the greater trochanter to slide past the coracoacromial arch.
The term “trumpet sign” describes the child’s typical pattern of bringing objects to the mouth (i.e., shoulder abduction accompanied by elbow flexion).
Posterior subluxation of the humeral head can develop as the internal rotators of the shoulder overpower the weaker external rotators and become contracted.
Mild shortening and atrophy of the limb are observed.
Biting of the fingernails and hands to the point of tissue damage is not infrequent (4.7%) in children with BPP and is more prevalent in children with total BPP.
The child should be reevaluated on a regular basis to ensure that scoliosis does not develop from muscle imbalance and asymmetrical motor patterns.
Causes
For many years, blame has been placed on the obstetrician when a neonate has been diagnosed with brachial plexus palsy (BPP). The assumption has been that the method of delivery and the traction applied to the head and neck during the birthing process cause the injury as the shoulder crosses the pubic arch. This theory has been supported by the fact that less than 1% of all BPP cases have been found in cesarean section deliveries.
In 2002, the American College of Obstetricians and Gynecologists recommended cesarean delivery for fetuses with an estimated weight of 5 kg or more, to reduce the prevalence of shoulder dystocia. If practitioners were to follow the recommendation, the effect on the cesarean delivery rate would be negligible, but the shoulder dystocia rate, which in this category of births is 20%, would be reduced.
In 2003, Raio and coworkers identified an increased incidence of brachial plexus injury among fetuses weighting more than 5 kg (2.86% vs 0.85% in fetuses weighing 4.5-4.599 kg), especially fetuses that developed shoulder dystocia. The authors suggested that when the estimated birth weight exceeded 4.5 kg, women should be informed of the increased risk of perinatal morbidity (including brachial plexus palsy) prior to making a decision on the mode of delivery.
Most neonatal BPP occurs in the birthing process. Risk factors for this type of injury, also referred to as obstetrical BPP (OBPP), include the following:
- Large birth weight (average vertex BPP, 3.8-5.0 kg; average breech BPP, 1.8-3.7 kg; average unaffected, 2.8-4.5 kg)
- Breech presentation
- Maternal diabetes
- Multiparity
- Second stage of labor that lasts more than 60 minutes
- Assisted delivery (e.g., use of mid/low forceps, vacuum extraction)
- Forceful downward traction on the head during delivery
- Previous child with OBPP
- Intrauterine torticollis
- Shoulder dystocia
The aforementioned study compared pregnancies and deliveries involving 45 infants with Erb’s Palsy with 90 controls. The risk for the condition was higher for children whose mother had gestational diabetes. Mothers who did not have gestational diabetes but who nonetheless gave birth to children with Erb’s palsy were found to have higher blood glucose values after a 50 g glucose challenge. Other variables found to be independently predictive of Erb’s palsy were a long deceleration phase of labor, a long second stage, high birth weight, and high neonatal or maternal body mass. Being a member of the black population also was found to be independently predictive.
Other, less common causes of neonatal BPP include the following:
- Neoplasm (e.g., neuromas, rhabdoid tumors)
- Intrauterine compression
- Humeral osteomyelitis
- Hemangioma
- Exostosis of the first rib
Diagnostic Considerations
Preplexus lesions are manifestations of the effects caused by the tearing of a rootlet, root, or spinal nerve that feeds the brachial plexus; these lesions may produce the same clinical findings as brachial plexus palsy, but electrodiagnostic testing can distinguish them from BPP lesions.
Cervical spinal cord injury (SCI) may be involved. Bowel and bladder function should be assessed carefully. Magnetic resonance imaging (MRI) of the spine should be performed in any child with bilateral BPP to rule out an associated SCI.
Patients with hemiparesis should demonstrate the presence of DTRs, an absence of apparent abnormalities in EMG findings, and an exaggerated (not a depressed) Moro reflex.
Patients with hypotonia of central origin should have preserved DTRs and an absence of findings on EMG.
Amyoplasiacongenita (a form of arthrogryposis) can be distinguished from BPP by rigidity of the joint and skin dimpling.
Children who have sustained humeral fracture demonstrate pseudoparalysis secondary to pain.
Anterior horn cell injury is unusual, but children with congenital varicella or congenital cervical spinal atrophy can present with a weak or flaccid arm accompanied by reduced sensation.
Laboratory Studies
Lab studies generally are not necessary for the diagnosis of brachial plexus palsy.
Imaging Studies
Until the advent of MRI, computed tomography (CT) myelography was the standard method for evaluating the integrity of the brachial plexus, and it remains arguably the most sensitive radiographic study to detect nerve root injuries. A water-soluble dye is injected intrathecally, and CT scans of the area in question are obtained. The main drawbacks to the procedure are radiation exposure, the need for sedation, a significant false-positive rate, and the lack of information on the distal brachial plexus. Some medical centers have abandoned the use of CT myelography, because direct observation during surgical exploration does not always correlate with CT myelographic findings.
High-resolution MRI is the best imaging study available for evaluating neonatal brachial plexus palsy. MRI requires no radiation exposure, is noninvasive, and provides more detail than does CT myelography. This test is most useful preoperatively to show the extent of trauma, including pseudomeningocele, and the presence of roots in the neural foramen.
While of little use in providing information on the anatomy of the brachial plexus, plain radiographs can be helpful in diagnosing hemidiaphragm paralysis from phrenic nerve involvement and fractures of the clavicle or humerus. Axillary radiographs also should be performed in children who show progressive loss of external rotation, to rule out posterior shoulder dislocation.
Other Tests
Electrodiagnostic studies are used as an extension of the physical examinationand can provide data on the severity and timing of the injury. The initial study usually is performed 2-3 weeks after injury, when signs of denervation are seen in children with moderate or severe injuries. Some authors feel that EMG provides useful information to track the reinnervation process and guide in surgical decision-making. Others feel that EMG does not provide prognostic information.
The examination typically includes the study of latencies of musculocutaneous and axillary nerves in Erb’s palsy. Incomplete injuries, motor and sensory nerve conduction studies (NCS) of median, ulnar, and, on occasion, radial nerves are performed. Sensory NCS are useful in discerning an avulsion injury; if the sensory nerve potential is intact in the context of a clinically insensate arm, an unfavorable prognosis is suggested. If respiratory distress was noted at birth, ipsilateral phrenic nerve conduction also is tested. Needle EMG is performed on muscles innervated by the affected nerve. In Erb’s palsy, these muscles include the supraspinatus, deltoid, infraspinatus, triceps, and biceps; in cases of total brachial plexus palsy, the muscles tested include those above, as well as the dorsal interossei and opponenspollicis.
Medical Issues/Complications
Aggressive forearm supination can lead to radial head dislocation. Unlike nursemaid’s elbow, radial head dislocation does not relocate easily in children with brachial plexus palsy (BPP) and can lead to a permanent elbow flexion contracture.
A small, but significant, percentage of children mutilate their fingers and hands as toddlers. Parents should be warned of this possibility, and they should take care to avoid cutaneous infection.
Without regular stretching, the child with residual weakness from BPP is at risk for progressive contractures, posterior shoulder dislocation, and agnosia of the affected limb.
Scoliosis can develop from muscle imbalance and asymmetrical motor patterns.
Complications
Children with brachial plexus palsy are at risk of developing complications, such as progressive contractures, bony deformities, scoliosis, posterior shoulder dislocation, and agnosia of the affected limb.
Prognosis
Statistics on children who attain complete recovery after brachial plexus palsy (BPP) vary widely, with reports ranging from 4-93%. This discrepancy is due, at least in part, to the time of evaluation. Many children present to the newborn nursery with temporary weakness (neurapraxia) that resolves prior to discharge and is thus unaccounted for in most of these studies.
Each child with more severe injuries presents with a slightly different degree of injury and responds differently to growth and therapeutic interventions. Experience in treating children teaches that children who present at 2 weeks of age with no signs of recovery generally are subject to development of sequelae, including mild scapular winging, inability to supinate the forearm fully, limitation in shoulder abduction, and forward flexion.
Peripheral nerves remyelinate at a rate of 1 mm/day. Thus, if the nerve is not transected, recovery can be expected by 4-5 months in Erb’s palsy, 6-7 months for an upper-middle trunk palsy, and 14 months for a total BPP. Some authors believe that infants who do not show signs of spontaneous recovery by 3-5 months usually are left with residual deficits if managed conservatively. Papazian and associates add that unfavorable functional outcome is related more often to aberrant reinnervation than to lack of reinnervation. Aberrant reinnervation is especially common in brachial plexus lesions secondary to the close proximity of the nerves involved.
As one might expect, findings consistent with a more extensive initial injury (Horner’s syndrome and total BPP) portend a less favorable prognosis. The converse also is true; children with isolated upper trunk lesions generally have a better prognosis. The presence or absence of phrenic nerve involvement does not appear to have prognostic value in BPP.
Yilmaz and coworkers compared MRI, electrophysiologic studies, and muscle strength scoring in 13 infants with BPP to determine which indicator provided the most accurate prognosis of the outcome at 1 year. They found that scoring of muscle strength (e.g., elbow flexion; wrist, finger, and thumb extension) was the most reliable measure, with 94.8% confidence at 3 months. Electrophysiologic studies, while helpful in identifying patients with an unfavorable prognosis, occasionally are overoptimistic (in 1 of 13 cases). MRI findings of pseudomeningoceles were seen in 2 of 5 patients with an unfavorable prognosis and in 2 of 8 with a good prognosis.
A study by Buitenhuis et al. found reduced sensibility of the thumb and index finger in children with neonatal BPP associated with C5 and C6, following either conservative or surgical treatment.
Michelow and colleagues retrospectively studied 66 children with BPP. They found that elbow flexion capacity at 3 months correlated well with good recovery of the arm at 1 year; however, predictions based on the presence or absence of elbow flexion at 3 months were incorrect in 12.8% of cases. When elbow flexion was combined with other physical findings (e.g., improved extension of the elbow, wrist, thumb, finger), the prediction was incorrect in only 5.2% of cases.
Eng and associates pointed out that most patients who are treated conservatively exhibit a return of biceps function. Biceps strength at 3-4 months, therefore, should not be the sole selection criterion for neurosurgical intervention. Many children did not show reinnervation of the biceps until 4-6 months of age, the forearm until 7-8 months, and the hand muscles (when affected) until 12-14 months. EMG signs of reinnervation are apparent 3-4 weeks before clinical recovery is seen. With conservative management alone, motor function continues to improve until age 2.5 years.
Eng classified sequelae as mild, moderate, and severe. Mild impairment was defined as minimal winging of the scapula, shoulder abduction to 90° or more, minimal limitation of shoulder rotation and elbow supination, normal hand function, and normal sweat and sensation. Moderate impairment included moderate winging of the scapula, shoulder abduction of less than 90° with substitution by other muscles, elbow flexion contracture, no supination, weak wrist and finger extensors, good hand intrinsic muscles, and some loss of sweat and sensation. Severe impairment was defined as marked winging of the scapula, shoulder abduction less than 45°, severe elbow flexion contracture, no supination, poor or no hand function, severe loss of sweat and sensation, or agnosia of the limb.
Outcomes were followed in 149 children whose cases were managed conservatively. A total of 4% recovered completely, 62% developed mild sequelae, 19% had moderate symptoms, and 15% sustained severe impairment.
In 1999, Waters performed a retrospective analysis of 94 children with BPP, comparing outcomes following neurosurgical repair, tendon transfers, osteotomy of the humerus, and conservative management. The investigators looked at 66 infants with BPP who presented in the first 3 months of life; 6 underwent microsurgical repair after no clinical improvement was seen in biceps contraction and antigravity strength at 6 months. Twenty-seven patients were referred after 6 months, secondary to persistent deficits; latissimus and teres major transfer was performed in 9 patients and a humeral derotation osteotomy in 7 for weakness in external rotation or tightness of the internal rotators.
The patients who underwent microsurgical repair had more favorable outcomes (based on the Mallet classification) than did those who did not have biceps function by 5 months, but their outcomes were not better than those who had a return of biceps function by 4 months. The Mallet scores, 4 in each category on average, after tendon transfers and osteotomy were equal to those of children who spontaneously regained biceps function in the first 3 months of life. Despite the small sample size, the investigators concluded that microsurgical repair leads to improved function in children who have not had clinical improvement of the biceps by 6 months. If biceps function by 3 months has been used as the main criterion for neurosurgical intervention, 39 additional patients would have qualified.
In 2004, Smith and colleagues concluded a 13-year prospective study that looked at the long-term outcome of patients with absent biceps function at 3 months. Of the 170 patients studied, 28 had absent biceps function at 3 months. Twenty-seven of the 28 had at least antigravity biceps function at the time of follow-up. The researchers concluded that patients with C5-C6 injury and absent biceps function at age 3 months often have good long-term shoulder function without brachial plexus surgery.
Fisher and associates studied the utility of elbow flexion as a criterion to recommend nonoperative treatment for obstetrical BPP. They studied 209 cases through retrospective chart review and organized them in 4 groups: Group A, no elbow flexion at 3 months (operative); Group B, elbow flexion at 3 months (operative); Group C, no elbow flexion at 3 months (nonoperative); and Group D, elbow flexion at 3 months (nonoperative). Elbow flexion was measured with the Active Movement Scale. The investigators found that Groups A, B, and C experienced a statistically significant increase in elbow function by the end of the study period (3 years), with no statistically significant differences between the 3 groups by the end of the study period. Group D showed statistically significant improvement by 3 months, compared with the other groups, leading most patients to be discharged from the clinic before the end of the 3-year study period.

Leave a Reply
Want to join the discussion?Feel free to contribute!